SJM’s Endosense Deal Highlights Growing Importance Of Contact Force Technology In AF Ablation
Executive Summary
St. Jude Medical has acquired Swiss company Endosense, a pioneer in the field of contact force sensing for atrial fibrillation ablation. The $170 million plus milestones deal will enable St. Jude to compete more effectively against market leader Biosense Webster/J&J, but it also emphasizes the growing importance of contact force technology in the AF ablation space.
The atrial fibrillation (AF) ablation market is one of the most promising medical device opportunities today, with procedures and device revenues growing at double-digit rates, an estimated worldwide market potential in the tens of billions of dollars, and market penetration currently standing at less than 5%. This common heart arrhythmia affects an estimated 15 million people world.wide, and that number could more than double in the coming decades as elderly populations expand.
But even though catheter-based AF ablation has consistently outperformed antiarrhythmic drugs (AADs) in recent studies and is rapidly emerging as a first-line therapy for patients with milder (paroxysmal) AF, the AF ablation device arena is still a long way from reaching its full potential. Existing catheter ablation devices have so far failed to address two of the biggest drawbacks hampering the space: the poor long-term durability of these procedures and their inability to effectively treat patients with longstanding, persistent AF. However, these shortcomings have opened up significant opportunities for companies with promising next-generation technologies, some of which could play a key role in driving growth in the AF device market in the months and years ahead.
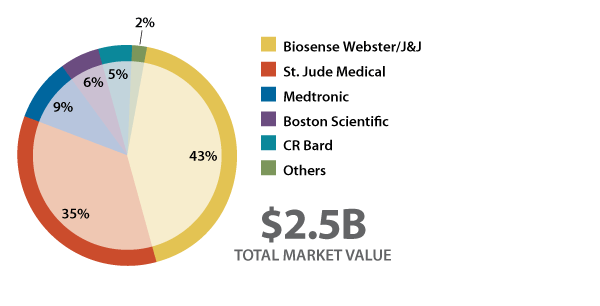
The future promise of new, innovative AF technologies clearly isn’t lost on the four large cardiovascular device companies that dominate the space: Biosense Webster Inc./Johnson & Johnson (J&J), which leads the catheter ablation market; St. Jude Medical Inc.; Medtronic PLC; and Boston Scientific Corp. (See Exhibit 1.) Over the years, all of these companies have made substantial investments to boost their AF ablation businesses, both via external acquisitions and internal research and development efforts, and that momentum is continuing.
Exhibit 1
Worldwide EP/AF Market, Share By Supplier, 2012
Larry Biegelsen, Wells Fargo Securities
In the past year alone, Boston Scientific bought arrhythmia mapping company Rhythmia Medical as well as CR Bard Inc.’s electrophysiology business to bolster its AF ablation offerings – the latter move will push Boston Scientific into the number-three spot in this field, ahead of current number-three player, Medtronic; and St. Jude Medical recently acquired Endosense SA, a pioneer in the field of ablation catheter contact force (CF) sensing technology. [See Deal][See Deal][See Deal] (Also see (Also see "Bard EP Deal Latest In Boston Scientific’s M&A Revival, Bolsters CRM Business" - In Vivo, 23 Jul, 2013.).) And there are likely to be more such deals in the future as strategic players look for new ways to strengthen their position in this high-growth space.
Innovation Abounds
Much of the recent innovation in the AF ablation arena centers on new arrhythmia mapping technologies, several of which are under development by innovative start-ups, including Topera Medical Inc., CardioInsight Technologies Inc., and Acutus Medical Inc. These companies are taking advantage of the latest advances in computer analysis and signal mapping technology to develop potentially groundbreaking new AF mapping systems. (See (Also see "New Paradigms Brewing In Atrial Fibrillation Ablation" - Medtech Insight, 23 Aug, 2013.), (Also see "At HRS, Topera Topples Conventional Wisdom About Atrial Fibrillation" - Medtech Insight, 23 May, 2013.) and (Also see "Device Companies Follow New Map To Atrial Fibrillation" - Medtech Insight, 23 Oct, 2012.).)
But work also is continuing on nearer-term advances aimed at making AF ablation procedures more effective and durable, and one of the most promising is CF sensing, designed to help ensure that physicians achieve optimum catheter tip to tissue contact and force during a radiofrequency (RF) AF ablation procedure. Too little CF may result in inadequate lesions and poor outcomes, whereas too much can injure the tissues and lead to serious procedure-related complications.
Although most of the work on CF sensing has involved pulmonary vein isolation (PVI) procedures, the current gold-standard technique for catheter-based AF ablation, the concept should apply regardless of where the ablation is being performed – whether around the PVs, on the roof of the atrium, or at some other electrical source point. And, it could be particularly useful for less-experienced operators and centers with lower AF patient volumes, helping level the playing field by providing a standardized feedback tool to guide optimum lesion creation.
Evidence Mounts In Favor Of Contact Force Sensing
Over the past several years, the clinical evidence has been piling up in favor of CF measurement, with studies showing that lesions created with CF guidance are more durable and result in better patient outcomes. And, CF could rapidly be adopted as the standard of care once the technology reaches the US market, a milestone that is expected to occur sometime next year.
The field of CF sensing was pioneered by Swiss company Endosense, with its TactiCath CF-sensing RF Ablation Catheter, which is CE marked and available in Europe and recently completed a pivotal US trial. As the trailblazer in this space, Endosense performed essentially all of the early heavy lifting, releasing the first abstract on CF sensing back in 2006, and since then, sponsoring a series of well-designed clinical trials that have established the positive impact of adequate CF on ablation lesion quality and procedural success. (See (Also see "Endosense: Facing Technology and Financing Challenges in AF" - In Vivo, 1 Mar, 2010.).) The company’s work includes the groundbreaking TOCCATA study, completed in 2010, which found that clinical outcomes improve when EPs deliver a CF greater than 20 grams, as well as its EFFICAS I post-market trial, which established a minimum Force Time Integral (FTI; a measure of force and time) of 400 gram-seconds for individual lesions.
Endosense Finds Itself In Right Place At Right Time
But the firm’s many hard-won accomplishments have attracted other players to this field, setting the stage for some fierce battles ahead. Of particular interest is recent progress by AF ablation market leader Biosense Webster Inc./J&J, which has developed its own CF-sensing ablation catheter, ThermoCool SmartTouch, and appears likely to edge out Endosense in the race to the US market.
Interestingly enough, J&J's progress in the CF space is turning out to be fortuitous for Endosense, which finally, after all the years of work and investment, has found itself in the enviable position of being at the right place (in terms of product development) at exactly the right time. On August 19, number-two ablation player St. Jude Medical announced it had acquired Endosense for $170 million up front plus an additional cash milestone payment of up to $161 million (See also (Also see "St. Jude Gains Force-Sensing Catheter Technology With Endosense Acquisition" - Medtech Insight, 19 Aug, 2013.).)
On August 19, number-two ablation player St. Jude Medical Inc. announced it had acquired Endosense for $170 million up front plus an additional cash milestone payment of up to $161 million.
The acquisition is important for St. Jude because it will enable the company to keep pace with J&J in the CF sensing arena. St. Jude has been developing a technology in-house called VeriSense that uses impedance to determine electrical contact between catheter tip and tissue, but doesn't measure actual contact force. The future of that technology is now uncertain, as studies in this field point to contact force as an essential parameter for determining lesion effectiveness. However, a St. Jude spokesperson commenting on the acquisition said that both the Endosense and VeriSense technology platforms provide "unique and compelling value" and that St. Jude plans to explore the future integration of the two technologies into a "streamlined, value-added solution." In the more immediate future, the acquisition will enable St. Jude to integrate Endosense's CF technology into its AF mapping system, EnSite Velocity, and potentially develop a MediGuide-enabled force-sensing ablation catheter as well, according to a company press release.
In the weeks prior to the acquisition announcement, Jan Keltjens, Endosense's president, CEO, and chairman (who will stay on as a consultant during the transition period), spoke at length about Endosense and its technology. He was upbeat despite J&J's recent progress, and given the deal with St. Jude, his positivity turned out to be justified. Endosense was well-funded prior to the acquisition, having raised a total of $44.6 million in a recent Series C round, and it was forging ahead with its US commercialization plans. The Series C officially closed last November at $40.3 million, with all the Series B investors participating, along with new investor NGN Capital, which led the round. Then, in March of this year, the Series C was topped off with an additional $4.3 million in funding from a new partner – the venture arm of an undisclosed strategic industry player (but not St. Jude Medical, sources confirm), a development Keltjens says was “a tremendous vote of confidence” in the firm’s technology.
According to Keltjens, the final TactiCath PMA module should be ready to file with the FDA during the fourth quarter of 2013, which means US launch could come in the second half of 2014. The company’s pivotal 300-patient US TOCCASTAR study reached full enrollment in June 2012 and one-year follow-up on these patients was completed this summer. The last bit of data for the PMA will come from a supplemental IDE study, opened in January to bring in the company's next-generation TactiCath Quartz device, and now nearing completion of patient follow-up, according to an Endosense spokesperson.
TactiCath Quartz features a number of product enhancements compared with the previous generation TactiCath, including a new force sensor designed to provide increased stability and precision and obviate the need for preprocedure calibration. The new system also has a “significantly smaller equipment footprint,” a more user-friendly graphics interface, and enhanced signal processing and digital output to facilitate connectivity with imaging devices and other equipment in the EP lab, the firm says. TactiCath Quartz was CE marked in June 2012 and launched in Europe last December.
The final TactiCath PMA module should be ready to file with the FDA during the fourth quarter of 2013, which means US launch could come in the second half of 2014.
According to Keltjens, Endosense recently completed the transfer of device manufacturing to a contract manufacturing partner and fully staffed its direct sales force in key European countries, including Germany, France, the UK, Benelux, and Switzerland. Moreover, the company made additional investments in its R&D pipeline and has been progressing well on the design of yet another generation of force-sensing catheter as well as “deploying some strategic 3-D imaging and mapping initiatives” via various collaborations. Some or all of those activities may shift now that St. Jude has the reins. However, Keltjens is convinced that the integration of information, such as that obtained through CF sensing, with advanced 3-D imaging tools, such as CT or rotational angiography, represents the future direction of this field.
Although Biosense Webster/J&J will likely reach the US market with a CF-sensing ablation catheter a few months ahead of Endosense/St. Jude Medical (the two companies began their IDE trials at about the same time, but because J&J already had an FDA-approved AF ablation catheter on the US market, it was required to perform only a single-arm nonrandomized supplemental IDE study, whereas Endosense needed a full IDE), Keltjens believes Endosense still has an edge from a technical performance point of view. “We think we have more consistent, more stable, easier to use, better forming force signals and catheter handling, so we’re not concerned about that,” he says.
And in all fairness, he points out, Endosense has pioneered the space. “We were the ones that thought of the concept of force sensing, materialized it in a commercially viable catheter, and we have done the heavy lifting in terms of generating the clinical data that gives it its staying power in the marketplace. And I think we’re now within spitting distance of being able to call this part of the standard of care,” Keltjens notes. “Biosense Webster recognized that potential and put all its marketing weight behind it.” But there are multiple ways to view that development, he says. J&J’s progress has “certainly sparked the interest of the strategics and validates the technology,” says Keltjens, who believes that in the rapidly growing AF space there is plenty of room for several companies to succeed.
In June, Biosense Webster/J&J submitted its PMA application for the ThermoCool SmartTouch device, and the company appears to be well-positioned to enter the US market in 2014.
Biosense Webster Awaits PMA Approval: Stage Is Set For Upcoming Battle
It may not be long before US physicians have a chance to weigh in on this issue. In June, Biosense Webster/J&J submitted its PMA application for the ThermoCool SmartTouch device, and the company appears to be well-positioned to enter the US market in 2014. As the current market leader in AF ablation catheters, the firm will likely hit the ground running once the device is approved, leveraging its existing installed base to convert US centers to the new tool. The ThermoCool SmartTouch catheter integrates with the company’s CARTO 3 navigation system and provides a real-time graphic display of both CF and tip force direction on the CARTO display screen during the ablation procedure.
In Europe, a bidirectional version of the ThermoCool SmartTouch was CE marked in December 2010 and a unidirectional model followed with a CE mark in February 2011. The devices reportedly have been doing well in the European market even though they are priced at a premium compared with standard ablation catheters. According to a company spokesperson, the US PMA application for the ThermoCool SmartTouch includes both the bidirectional and unidirectional models, so when the company receives FDA approval, US physicians will be able to choose the type that they prefer.
Twelve-month outcomes from SMART-AF, the company’s single-arm US IDE trial of the ThermoCool SmartTouch, were presented during a late-breaking session at this year’s Heart Rhythm Society (HRS) meeting by Andrea Natale, MD, executive medical director of the Texas Cardiac Arrhythmia Institute, St. David’s Medical Center. SMART-AF enrolled 172 symptomatic PAF patients at 21 US centers; 122 patients who were treated following the roll-in phase were included in the effectiveness cohort for analysis.
The study met both its safety and efficacy endpoints, with a 9.9% adverse event rate (there were no unanticipated device-related adverse events) and 72% of patients demonstrating freedom from symptomatic AF or other atrial arrhythmias at 12-month follow-up. A Kaplan-Meier analysis showed that procedural success was 15% higher (77% vs. 62%; p = 0.03) when CF was kept within a prespecified range at least 80% of the time (n = 52) than when it wasn’t (n = 56). And the success rate increased even further (83.7% vs. 60.7%; p = 0.011) when CF was within range over 82% of the time. (See Exhibit 2.) Patients in this group also were more likely to be free from recurrence at follow-up. The strategy also proved safe: staying inside the desired CF range had no detrimental impact on the study’s safety outcomes.
Exhibit 2
Results Of The SMART-AF Trial; Contact Force Correlated With Primary Effectiveness
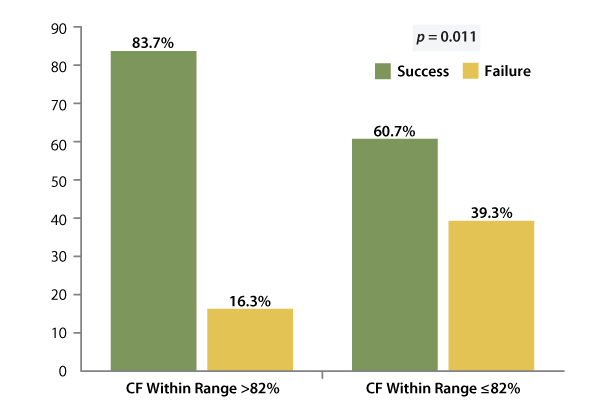
Andrea Natale; from a presentation at the 2013 HRS meeting
During a press conference discussing the results, Natale remarked that CF measurement is likely to be considered standard of care for AF ablation in the future, although the issue of cost might play into that, because incremental technology improvements cost more. For now, however, US EPs appear to be enthusiastic and eagerly awaiting the release of these devices in the US. Although some very experienced operators may wonder whether or not they need CF measurement in every case, Endosense’s Keltjens says the data have proven it’s not a trivial piece of information. Tactile feedback doesn’t correlate well with the actual pressure being applied, he points out, and one of the big findings from the EFFICAS study is that “it’s not about average lesion and average force – every single lesion has to be right.”
This article was adapted from the August issue of Medtech Insight.