Immunotherapy: Big Pharma’s Seductive Embrace
Executive Summary
The oncology community believes that immunotherapy will be foundational, in the same way that combination chemotherapy became the backbone of treatment in the 1970s. The marching order is to trial broadly, quickly, making clinical development particularly well-suited for Big Pharma.
- It’s early days in the understanding of which patients will respond to immuno-oncology drugs. The marching order is to trial broadly, quickly, making development particularly well-suited for Big Pharma.
- The major Big Pharma players are setting up combination trials early in development with additional immunotherapies and targeted agents. This notion of seeking synergy from the get-go is a dramatic shift in how cancer drugs are developed, with strong implications for partnering, pricing, and life-cycle management.
- Whether the approach is diversified or focused, in most cases an effective partnering strategy will be key to establishing a market. That’s good news for a variety of companies including those with discovery platforms, immuno-oncology assets that have some proof-of-concept, and targeted agents that combine to boost efficacy.
- Not all companies need enter the fray, but an awareness of how their oncology drugs pair up with immunotherapies is as essential as was factoring in the use of chemotherapy a generation ago.
The emergence of Merck & Co. Inc.’s Keytruda (pembrolizumab), granted an accelerated FDA approval in September 2014 based on data from more than 400 patients in an initial Phase I study started in early 2011, makes a compelling argument for Big Pharmas with the resources to rapidly develop fast followers to enter the race to commercialize cancer immunotherapies. Companies are doing just that, in many cases setting up combination trials early in clinical development, either with additional immunotherapies or with targeted agents.
Merck followed in the footsteps of Bristol-Myers Squibb Co., whose Yervoy (ipilimumab), approved in 2011, validated decades of effort in immuno-oncology. BMS’ second entry into the field, Opdivo (nivolumab), like Keytruda, inhibits PD-1, a molecule that stops T-cell activation and in so doing inhibits T cells from attacking a tumor. Opdivo is approved in Japan and by all accounts will be given the go-ahead from FDA in the next few months.
To a large extent, the promise of improved overall survival rates through checkpoint modulation, along with the many unknowns around which checkpoints are active in which patients and which tumor types, has made immuno-oncology a Big Pharma game – and a seductive one that competitors and collaborators alike are embracing. Larger firms have resources to place a competent molecule – one that has proven mechanistically sound – into a large early-stage trial and then advance it into as many clinical indications as possible to best understand how to apply it to treatment. That makes immuno-oncology a field where partnering strategies are paramount – both for companies with discovery platforms yielding candidate molecules and for the pharmas wanting to run with them. Combination regimens involving immunotherapies should also open up commercial opportunities in niche markets that smaller companies can pursue.
A Unique Benefit
Yervoy’s showing that a percentage of patients benefit from the drug for years – and that even some patients who could not tolerate more than one dose have a sustained response – opened the door for the PD-1s.
PD-1 drugs, including antibodies that target PD-1 itself and its main binding partner PD-L1, and CTLA4, the target of Yervoy, are the first generation of immune checkpoint targets. Roche and its Genentech Inc. unit are in late-stage trials of an anti PD-L1 antibody, MPDL3280A, led by a Phase III study in non-small-cell lung cancer (NSCLC), while AstraZeneca PLC and its MedImmune LLC division are pursuing NSCLC with its Phase III anti-PD-1 MEDI4736. AstraZeneca/MedImmune are also continuing to test the anti-CTLA4 tremelimumab. Meanwhile, Novartis AG made a big splash in 2012 when it added chimeric antigen receptor T-cell (CAR-T) technology through an alliance with the University of Pennsylvania and more recently, obtained checkpoint inhibitors not yet at the clinical stage, including an anti-PD-1, through its acquisition of CoStim Pharmaceuticals Inc.[See Deal][See Deal] And Pfizer Inc., which had partnered with Merck for access to clinical stage immuno-oncology candidates to test in combination with its oncology portfolio, has now teamed up with Merck KGAA on development of the latter’s MSB0010718C anti-PD-L1 antibody. [See Deal] (See (Also see "Pfizer Seizes Long-Term Immuno-Oncology Opportunity With Merck Deal" - Pink Sheet, 17 Nov, 2014.).)
Prior to the Yervoy approval, investment by Big Pharma in immuno-oncology had been tentative, as failures with strategies involving therapeutic vaccines and tumor-infiltrating lymphocytes mounted. BMS was the exception, having doled out approximately $2 billion in cash in 2009 to acquire Medarex Inc., which gave it both Yervoy (for which it had already entered into a development agreement) and Opdivo. [See Deal][See Deal]
Yervoy was the first drug to show a survival benefit in melanoma; now, the PD-1s are known to be active in several solid tumors – NSCLC, renal cell carcinoma, and melanoma most prominently. Data are encouraging in ovarian, head and neck, bladder, and hematologic malignancies and the drugs may well show survival benefits in some patients beyond what are seen with Yervoy. Generally, the response rates are slightly upwards of one-quarter of patients: anticipating which patients will respond to the drugs and which will not is problematic, however.
“You are seeing improvement in survival that is way more than modest,” says Daniel Afar, PhD, head of development for the Abbvie Biotherapeutics division of AbbVie Inc. (Afar was previously at Merck, where he led several biologics programs including Keytruda’s.) Even though cancer is still present, it is being held in check.
Indeed, holding a tumor at bay is a key differentiating characteristic of checkpoint inhibitors. With chemo and targeted agents, cancer cells inevitably develop mutations or drug-resistant clones. In general, that happens fairly rapidly, within six to 12 months. “You may have improvement in survival but almost always, the tumors come back,” Afar says. Unlocking a checkpoint, on the other hand, allows T cells to recognize the tumor more effectively and for a longer time.
Trial Quickly And Broadly
The leaders in checkpoint blockade – BMS, Merck, Roche, and AstraZeneca – are conducting a broad swath of clinicial trials, often using combinations with targeted agents or chemotherapy. (See Exhibit 1.)
Exhibit 1
Combination Trials Of Leading Checkpoint Inhibitors
|
Drug (Company) |
Total Trials* |
Combo Trials |
Proportion |
|
Yervoy (Bristol-Myers) |
42 |
26 |
62% |
|
Keytruda (Merck) |
32 |
16 |
50% |
|
Opdivo (BMS) |
25 |
13 |
52% |
|
MEDI4736 (AstraZeneca/MedImmune) |
19 |
14 |
74% |
|
MPDL3280A (Roche/Genentech) |
12 |
6 |
50% |
|
Tremelimumab (AstraZeneca/MedImmune) |
11 |
10 |
91% |
*Includes trials started after January 1, 2013.
SOURCES: Bionest; clinicaltrials.gov
It’s a revolution in the treatment of cancer to be able to go this quickly and broadly, which does not necessarily mean culling huge numbers of patients as much as focusing on a number of tumors as early on as possible – a strategy amplified by the numbers of possible combinations to test.
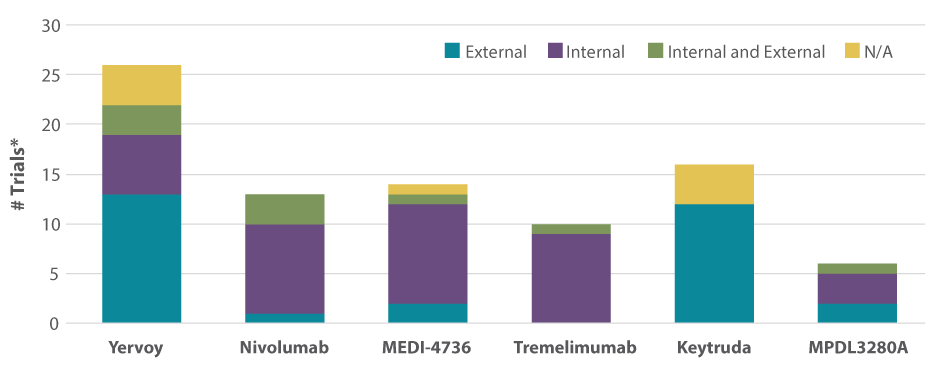
BMS did that early on with Opdivo, investing and focusing its pipeline with multiple studies. (See Exhibit 2.) The company pursued several tumors extensively: lung, melanoma, and RCC. Having Yervoy also gave BMS an edge, and the ability to combine with the rest of its immuno-oncology pipeline “can’t be undervalued,” says Jamie Foley, former global commercial lead, Nivolumab and Nivolumab + Yervoy for BMS and now Bayer HealthCare AG.’s Global Marketing Oncology Lead for its late-stage pipeline assets. It increased the depth of knowledge and experience in immuno-oncology.
BMS has been developing additional checkpoint inhibitors – the antibodies lirilumab (anti-KIR) and urelumab (anti-CD137), which are now in combination trials with Opdivo. It also just started Phase I with lirilumab and urelumab combined with the Abbvie drug elotuzumab in myeloma. While still part of Abbott Laboratories Inc., Abbvie acquired elotuzumab when it bought Facet Biotech Corp. [See Deal][See Deal]
Exhibit 2
Combination Approach To Clinical Trials
*Trials started after January 1, 2013.
SOURCES: Bionest; clinicaltrials.gov
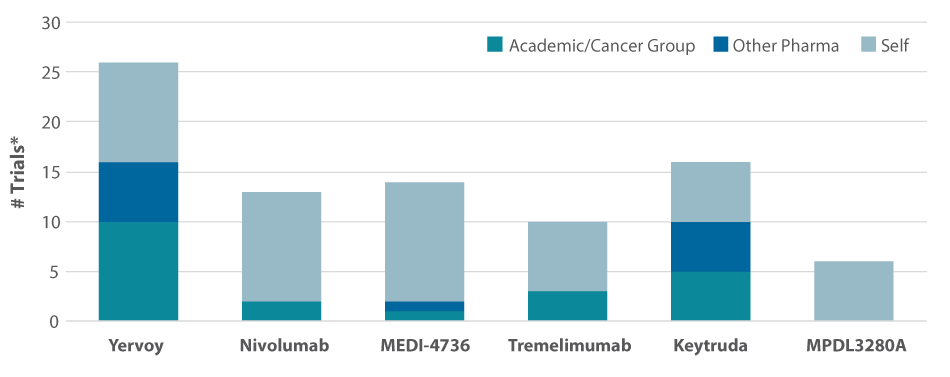
When so much is unknown, going broad, quickly, with a pipeline of checkpoint blockage candidates used in combinations helps match the best regimen to a patient population. Combination therapy also means joining immuno-oncology assets with the standard of care, including targeted therapies. Merck put in place the right partnerships to make up for the fact that it didn’t have a pipeline of targeted agents. Roche, more like BMS than Merck, tapped its pipeline and is focused more internally. (See Exhibit 3.) Depending on other internal assets, a company may take more of a diversified or a focused approach. Either way, however, partnering will be an important element of strategy. Partnering can enable progress faster. Plus, no company can have all varieties of immuno-oncology assets within its own portfolio.
Exhibit 3
Trial Sponsor Breakdown
*Trials started after January 1, 2013.
SOURCES: Bionest; clinicaltrials.gov
That gives biotechs a significant amount of room to get into the game, especially since plenty of potential partners are willing – and waiting – to bear the development costs. Nor does it take that much to establish an asset’s value, as evidenced by Merck KGAA’s sale of a share of its PD-L1 to Pfizer, with barely anything beyond proof-of-concept ongoing in Merkel cell carcinoma. “I don’t think the biotechs have to do all that much to show an initial proof-of-concept for potential partners to be interested,” Foley says.
How times have changed. “Companies who wouldn’t talk [about immunotherapy] in the Medarex days are now claiming to be world experts,” says Tibor Keler, PhD, EVP and chief scientific officer at Celldex Therapeutics Inc., the former vaccines division spun out of Medarex. [See Deal]
“The level of competition that has come about because of the [BMS and Merck] successes is pretty clear,” he says. That changes the approach companies can take – especially for a small biotech company, knowing the resources Big Pharma can throw at these programs.
Celldex currently is collaborating with BMS on a combination of its varlilumab (anti-CD27 checkpoint) with Opdivo. (See (Also see "Celldex Differentiated Immuno-Oncology Asset Ripe For Combos" - Pink Sheet, 7 Jul, 2014.).) “We don’t think we need the fourth or fifth PD-1 blocking strategy” to combine with its immuno-oncology approaches, Keler says, “as long as we can find partners that are willing to have the right type of collaboration agreement.” In the end, he does not think Celldex will have to give up control of its assets – the standard pharma-biotech licensing model. In general, pharma is “a lot more flexible than in the past” in this regard, he says.
The New View Of Combinations
Even before its collaboration with Merck KGAA, Pfizer had established collaborations with external partners with PD-1 combinations. But Pfizer Oncology had also publicly stated it was interested in developing its own PD-1 therapy to be able to create the best possible combination with a PD-1 agent. Not having a coherent internal program to optimize combination regimens, “would not be the best way to go forward,” says John Lin, PhD, VP, immunology. Whereas several of the agents have shown activity in lung and melanoma, “we were not certain whether there were differences in these drugs,” he says. Medivation Inc. recently acquired CureTech Ltd.’s Phase II PD-1 pidilizumab, perhaps contemplating combination therapy with its prostate drug Xtandi (enzalutamide). [See Deal] Curetech got rights to pidilizumab back from former partner Teva Pharmaceutical Industries Ltd. in 2013. [See Deal]
Data with the PD-1’s are being generated in diseases previously characterized as non-immunogenic, such as NSCLC, head and neck cancer, and bladder cancer – conditions that a few years back would not have been considered for immunotherapy. “I would expect it will become the core modality for treating cancer patients,” says Axel Hoos, MD, PhD, VP, oncology R&D at GlaxoSmithKline PLC (GSK). “A company that’s really making a large effort in oncology and tries to have a diversified portfolio cannot avoid immunotherapy,” he says. Hoos was an early champion of immunotherapy while at BMS prior to GSK, where he worked on Yervoy’s development with its inventor, Jim Allison, PhD, of the MD Anderson Cancer Center.
The level of complexity in achieving immune intervention successfully is higher than for most other cancer therapies. For example, for anti-PD-1 to work, immune cells – specifically T cells – have to reside within the tumor. A tumor’s inflammatory state will also dictate a PD-1’s effectiveness. Melanoma, for example, seems to be in a constant inflammatory state with a huge immune cell infiltration. The T cells are already present throughout. But in other cancers, the immune cells do not penetrate: they are all on the periphery of the tumor. Hence the need for other checkpoint inhibitors, knowing that the immune system has enough T cells with specificity for a tumor to kill it if they are not exhausted – if you re-activate them and if there is no barrier to their going into the tumor.
More specific checkpoint inhibitors may provide a better response in certain patient populations with or without a PD-1. Plus, if the tumor is overtaken by macrophages or myeloid cells, blocking PD-1 or PD-L1 might not work.That leaves considerable room for regimens comprising combinations of PD-1s, next-generation checkpoint inhibitors, other modalites of immunotherapy such as dual targeting antibodies (bispecifics) and engineered T-cell receptors, and targeted agents. “Even given the flurry of recent dealmaking, the space is wide open,” Hoos says.
There is an element of market segmentation that comes in by combining immunotherapy with targeted therapies in the population where the targeted therapy works.
Importantly, there is an element of market segmentation that comes in by combining immunotherapy with targeted therapies in the population where the targeted therapy works. Mutated melanoma is one obvious example: combining a PD-1 inhibitor withZelboraf (vemurafenib) or GSK’s Tafinlar (dabrafenib). “Based on the combinations being pursued, I would see that segmentation continuing,” Hoos says. Segmentation could also be driven by finding patient populations that so far have not responded to immunotherapy. “It’s probably not that patients cannot respond,” says Hoos. “It is a matter of what mechanism we are using to engage the immune system.” PD-L1 expression might be one aspect for segmentation, but may be just the tip of the iceberg. “With time, as we build our biomarker story with immunotherapy, we will see some segmentation there as well,” he says.
Playing Catch-Up
If a company is late to the immuno-oncology race, a range of strategies can be applied to compete with the leaders and carve out a marketable space. Taking the long view and bringing several different modalities into a portfolio is one approach. The recent approval of Amgen Inc.’s first-in-class bispecific antibody Blincyto (blinatumomab) less than two months after FDA accepted the company’s Biologics License Application (BLA), is a testament to the potential for fast introduction of additional immuno-oncology approaches. (See sidebar, “Amgen Gets The First BiTE.”)
GSK charged Hoos, who left BMS just under three years ago, with setting a strategy for the firm to catch up in immuno-oncology and differentiate itself. GSK is placing bets on different modalities that can either work together or co-exist and not necessarily compete directly with what’s already been done around PD-1 checkpoint modulation. Its first deal was with MD Anderson – playing on Hoos’ prior relationship with Yervoy inventor Jim Allison – on a checkpoint modulator directed to OX-40 activating antibodies. [See Deal] GSK has also accessed an autologous T-cell receptor therapy targeting NY-ESO-1 (also the target of Celldex’s CDX1401) through an agreement with Adaptimmune Therapeutics PLC and antibody-targeted T-cell receptors (ImmTACs) via a license with Immunocore Ltd.[See Deal][See Deal]
Abbvie, which is still at the preclinical stage in immuno-oncology, is also not looking to directly compete with a PD-1, but is developing other checkpoint inhibitors that keep the immune system in check.
Abbvie may gain leverage from its pipeline of targeted agents including a BCL-2 inhibitor, ABT-199, which induces cell death. Applying a targeted agent along with an anti-PD-1, “gives you a competitive edge over a company that doesn’t have it if you’re going into the same indication,” says Afar, echoing Hoos’ vision of how the oncology market will segment.
Using ABT-199 to induce cell death leads to antigens floating around that can then be offered by antigen presenting cells to T cells and can prime those cells. Then, once activated, anti-PD-1 could have an effect.
“You have the apoptotic activity of ABT-199 but also the immune activity targeting the tumor as well,” Afar says. That principle could apply to just about any cancer drug including an antibody-drug conjugate (ADC) or even a conventional chemotherapeutic that induces cell death. Indeed, many ADCs were dropped because of a marginal response, but if they were combined with an immunotherapy there could be useful synergy because of this vaccination effect.
It’s possible to enter the immuno-oncology drug development race on a relatively small budget. “You won’t have as many shots on goal, but you can be strategic.” - Daniel Afar, PhD, Abbvie.
Further, it’s possible to enter the immuno-oncology drug development race on a relatively small budget. “You won’t have as many shots on goal, but you can be strategic,” Afar says, if, for example, you have data that say an asset is active in PD-1-refractory patients. “That’s the development plan. Maybe the indication will be third-line melanoma, not commercially attractive, but it will tell you if the drug is active.”
A study to establish proof-of-concept in Phase I – just as anti-PD-1 showed in refractory patients, with a 30% response rate across indications – is far from financially onerous. But a small biotech might not have the wherewithal to go all the way to approval, because the goal would be to go into earlier lines and try out multiple indications. “You’d have to partner. It will be very hard for a small company to be a big player,” he says. “To be a big player I think you need to have a big budget.”
Tapping into a discovery platform to make many small bets quickly is another route of entry. Bayer, for example, took a license to two of drug discovery specialist Compugen Ltd.’s preclinical checkpoint inhibitors in 2013, which the pharma would take into and through the clinic. [See Deal]
The largest deal of this type came 14 months later, when Celgene Corp. saw the potential to move quickly and broadly into the clinic with Sutro Biopharma Inc. They agreed to develop up to six bispecific antibodies and/or ADCs directed at immuno-oncology targets, including but not limited to checkpoint pathways. [See Deal] Sutro’s rapid design capabilities using cell-free protein synthesis speeds the preclinical process, allowing quick reengineering of leads. That’s especially valuable in an area where the science is moving fast. “If we see new concepts being suggested or precedented as combinations or monotherapies, we can leap on that in a matter of weeks to be able to interrogate the preclinical models to see if that makes sense,” says Trevor Hallam, PhD, Sutro’s chief scientific officer.
“These therapies have only been in the clinic for a dozen years, and started with only crude measures for how they work,” he says. The thinking has been that if the tumor gets bigger in four to six weeks, treatment should stop. “But it is quite clear that is a natural part of the mechanism because as that tumor gets swollen with the immune system attacking it with reactivated immune cells, it will look a whole lot worse before it gets better,” he says. “A lot of clinical studies were terminated early: if they had continued, there might be more interesting therapies that just CTLA4 and PD-1,” he says. “We’ll see if those things re-emerge.”
Value Split Is The Ultimate Issue
The field has evolved sufficiently that companies can think differently about development and partnering, by factoring in early the potential for combining with an immunotherapy. “From the beginning [of a program], we foresee how we can develop a targeted therapy and prolong the response in some patients using an immunotherapy, in a sequential approach or in a combined approach,” says Pascal Touchon, VP, business development and scientific cooperation for Servier SA.
The French firm has deals with Cellectis SA around CAR-T targets and MacroGenics Inc. on bispecific antibodies including an inhibitor of B7-H3, a checkpoint target that is expressed differently in tumors than PD-1 or CTLA4. It therefore might be amenable for development in a specific group of patients within a particular indication using trial enrichment and biomarker approaches. [See Deal][See Deal][See Deal] “Sharing risk and investment, we think that as a midsized company we have the possibility to address different types of approaches and indications,” Touchon says.
A company without an immunotherapy in its portfolio has opportunity if it has the right partnering strategy to make the necessary combination studies, he says. And that raises questions of pricing. “One of the question marks we have before we go into combining these therapies is how that would be accepted by the payors,” he says. “That is going to be a key issue for somebody with only a targeted therapy.”
A variety of scenarios could play out. In one case, introducing a combination with a currently approved PD-1 agent with its price already set could mean payors will look at the total cost of intervention – “and then pressure you to make that acceptable, whatever the reimbursement system is,” Touchon says. In that case, the pressure will be on the second agent at least as much as on the anti-PD-1.
Given the rapid expansion of combination trials involving checkpoint inhibitors, it soon might be challenging to carve out space. But it might be possible to select a group of patients in which to show efficacy combined with a targeted therapy, and then the particular indication would be obtained for that duo – possibly extending the life cycle of the targeted agent. “That would create investment opportunities in as many indications as are being developed right now. With the need to agree on how to share the return,” says Touchon.
The power of negotiation could also lie with the targeted agent, assuming a greater level of efficacy when combined with an anti-PD-1. If there are several PD-1s available, being associated with a particular PD-1 in a combination could create a market for that PD-1, especially in a new indication or a new patient population. “With a good targeted therapy, you could go to several PD-1 companies and decide which one allows you flexibility in terms of the payor strategy,” says Touchon. A company with a new targeted therapy could also develop its own anti-PD-1 for approval exclusively for its set of indications.
Differentiating Cell Therapies, And Not
The value proposition for CAR-T and other methods for introducing T cells or T-cell receptors is vastly different. The technologies cannot be ignored for several reasons: the publication of efficacy seen in leukemia in the University of Pennsylvania CAR-T program, its sustained effect as a one-time monotherapy, and the fact that checkpoint inhibitors are bound to fail in many patients.
Indeed, some tumors have very few mutations and not enough immunological differences for T cells to recognize them. That said, a checkpoint inhibitor won’t be doing much good if there is no immune response to activate at the tumor – in patients undergoing chemo and/or radiation therapy, whose immune systems are severely compromised.
“For those patients we believe we have to give them more T cells,” says Pfizer’s John Lin – specifically anti-tumor T cells that can recognize well-defined and important tumor antigens. But the tumor may still resist the T-cell infusion. “So it is reasonable to speculate at this point that we could also combine anti-PD-1 antibodies with CAR-T cells,” he says. Pfizer has a 15-target collaboration with Cellectis to use the latter’s allogeneic CAR-T technology. [See Deal]
Although pricing may be in a different ballpark – several hundred thousand dollars for CAR-T versus perhaps $100,000 for checkpoint-plus combinations – the value split may still have to be addressed. “We are convinced that combination trials are compulsory for CAR-Ts,” says André Choulika, PhD, CEO of Cellectis, perhaps including bispecific antibodies.
“Oncology is a field built on combination therapy, but for now we believe that monotherapy using cells has enough efficacy to justify the benefit/risk that will be important to the regulatory approval,” says David Chang, MD, PhD, chief medical officer of Kite Pharma Inc., a Cellectis competitor developing autologous CAR-T. (See (Also see "Deal-Making, Financing Coalesce Around Promising CAR-T Space In Cancer" - Pink Sheet, 8 Sep, 2014.).) “That’s where our focus is and combinations will follow soon after.”
The focus on monotherapy may hold for seeking approval, but medical practice is another matter. Just as combination regimens have evolved for conventional chemotherapy, then targeted agents, and now checkpoints, the wise bet is it will be so for all immunotherapies for the next generation, at least.
Olivier Lesueur ([email protected]![]() ) is a managing director with Bionest Partners, based in New York and Paris, and Rachel Laing ([email protected]
) is a managing director with Bionest Partners, based in New York and Paris, and Rachel Laing ([email protected]![]() ) is a manager with Bionest in New York. Mark Ratner ([email protected]
) is a manager with Bionest in New York. Mark Ratner ([email protected]![]() ) is a freelance writer.
) is a freelance writer.