Biopharma Quarterly Dealmaking Statistics, Q1 2015
A look at financing, M&A, and alliance activity January–March 2015
Executive Summary
Biopharma financing reached $15.1 billion, with record-breaking FOPO dollar volume making up close to $10 billion of the total; AbbVie topped the $66.5 billion M&A activity with its $20 billion buy of Pharmacyclics; and gastroenterology was one of the most highly partnered therapeutic areas.
Financings
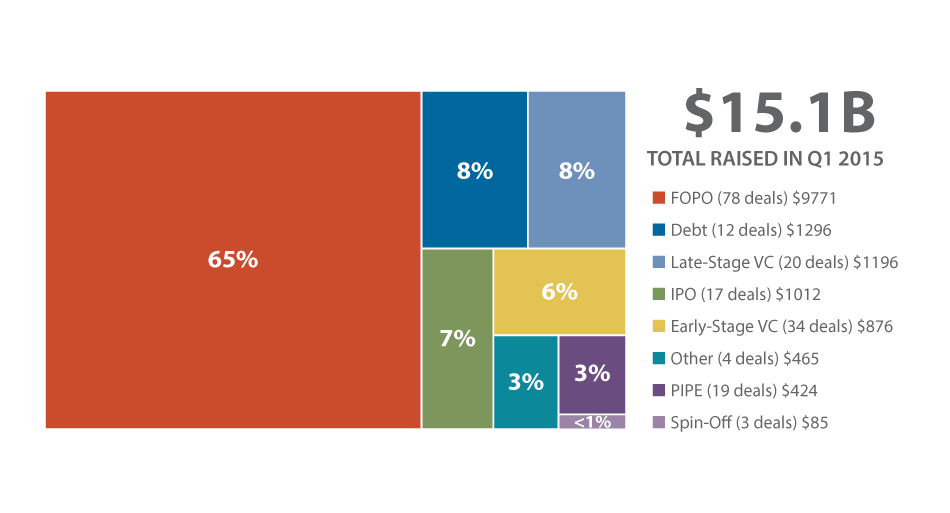
Representing a 72% increase over Q4 2014, first-quarter 2015 financing totaled $15.1 billion and featured 187 transactions (52 more than Q4). (See Exhibit 1.)
Exhibit 1
Q1 2015 Biopharma Financing
By Deal Type ($M)
Strategic Transactions
Follow-on offerings were the leading deal type this quarter, making up 65% of the fourth-quarter financing total through an astounding 78 transactions, which brought in nearly $10 billion. This is the most ever brought in by this financing type in a single quarter, at least in recent years. (The next-highest aggregate dollar amount was $3.9 billion in Q1 2014.) (See Exhibit 2.) The average deal value was $125 million, and 33 of the 78 deals met or greatly exceeded $100 million in proceeds.
In Q1’s richest FOPO, Valeant Pharmaceuticals International Inc. netted $1.4 billion [See Deal], or 15% of the FOPO category total. Hepatic- and metabolic disease-focused Intercept Pharmaceuticals Inc. brought in an aggregate $533 million through February [See Deal] and March [See Deal] FOPOs. In Q2 2015, the company expects to file for US marketing approval of its lead candidate INT747 (obeticholic acid) in primary biliary cirrhosis. According to BioMedTracker, INT747 has a 97% likelihood of approval (10% above average).
Exhibit 2
Record-Breaking Q1 2015 FOPO Dollars
Strategic Transactions
With more and more companies attempting to secure a hefty chunk of money to support substantial acquisitions or alliances, debt financings also played a role as a non-dilutive funding option in the Q1 financing scene. Through the sale of $575 million in senior secured notes to Deerfield and Pharmakon affiliates [See Deal], Depomed Inc. had the largest, funding its January US license to Johnson & Johnson’s Janssen Pharmaceutical Cos. division Janssen Pharmaceuticals Inc.'s Nucynta (tapentadol) pain products. [See Deal]Horizon Pharma PLC netted $387 million in a March convertible debt offering [See Deal] to help with its $960 million buy of US orphan drug firm Hyperion Therapeutics Inc. [See Deal]
Venture rounds including corporate VC investments made up 61% of the total venture transactions – approximately $1.3 billion of the $2.1 billion total. Although CVCs invested the most dollars ($657 million) into Series C rounds, they also backed 14 early-stage rounds. (See Exhibit 3.) Novartis put money into early-stage rounds for five different biotechs across various therapeutic areas, and in addition, signed an alliance with newly formed metabolic disorders firm Semma Therapeutics [See Deal], concurrent with the start-up’s $44 million Series A round. [See Deal] Eli Lilly & Co. backed six biotechs in five A and B rounds – two of which Novartis also participated: Aeglea Biotherapeutics Inc.'s $44 million Series B [See Deal] and Surface Oncology’s $35 million Series A [See Deal] – as well as a $100 million Series C raise by Innovent Biologics Inc. [See Deal] Lilly, along with Pfizer Inc. and SR One, also took part in Nimbus Therapeutics’ $43 million Series B financing. [See Deal] As the company transitions from a discovery company to one developing treatments for non-alcoholic steatohepatitis (NASH), it will put the cash toward advancing its lead program, an acetyl coA carboxylase (ACC) inhibitor, into clinical development – representing the first allosteric and liver-targeted inhibitor intended for the treatment of NASH. (See (Also see "Nimbus Thinks It Has “Non-First-Mover” Advantage In NASH" - Pink Sheet, 20 Mar, 2015.).)
Exhibit 3
Corporate VCs Pour $1.3B Into Q1 2015 Venture Rounds

Strategic Transactions
Four-year-old messenger DNA start-up Moderna Therapeutics LLC raised $450 million in Series C funding [See Deal] from 43 investors, including repeat corporate backers AstraZeneca PLC and Alexion Pharmaceuticals Inc., completing the largest biotech venture round to date![]() . Moderna, rich with $800 million in cash, has 45 preclinical programs in development across a variety of therapeutic areas and boasts alliances with Merck & Co. Inc. [See Deal], Alexion [See Deal], and AZ. [See Deal] Although corporate VC participation was plentiful during Q1 and these Moderna deals are an exception, a recent study by START-UP (see (Also see "Corporate Venture "Parents" Rarely Buy Or Partner With Biotech Investments" - Scrip, 8 Apr, 2015.)) indicates that these corporate venture investments in fact rarely result in a partnership with or acquisition of the smaller biotech.
. Moderna, rich with $800 million in cash, has 45 preclinical programs in development across a variety of therapeutic areas and boasts alliances with Merck & Co. Inc. [See Deal], Alexion [See Deal], and AZ. [See Deal] Although corporate VC participation was plentiful during Q1 and these Moderna deals are an exception, a recent study by START-UP (see (Also see "Corporate Venture "Parents" Rarely Buy Or Partner With Biotech Investments" - Scrip, 8 Apr, 2015.)) indicates that these corporate venture investments in fact rarely result in a partnership with or acquisition of the smaller biotech.
IPO dollars at $1 billion were less than half of the previous quarter’s $2.1 billion total (the second-highest moneymaking category of Q4). However, Q1 deal activity remained plentiful with 17 IPOs, only five fewer transactions than Q4’s 22, and there are still eight Q1 filers whose offerings have yet to price. Nine of the 17 completed IPOs – accounting for $487 million of the Q1 total – were done by European companies, led by French cancer immunotherapies firm Cellectis SA, which netted $212 million through its debut of American Depositary Shares on Nasdaq. [See Deal]
Acquisitions
The first-quarter 2015 biopharma merger and acquisition activity reached an incredible $66.5 billion from 38 deals, capped off with three gigantic acquisitions by AbbVie Inc., Pfizer, and Valeant Pharmaceuticals. Of the 38 deals, eight multibillion-dollar transactions made up 92% of the Q1 M&A total. (See Exhibit 4.) Transactions by Big Pharmas, which reached $19.2 billion (with an average deal value of $1.9 billion), accounted for 29% of the aggregate M&A dollars.
Exhibit 4
Top Biopharma M&As, Q1 2015
|
Date |
Acquirer/Acquired (Business) |
Terms |
|
March |
AbbVie/Pharmacyclics (cancer drugs) |
$19.86B ($11.52B in cash and $8.34B in AbbVie equity): $261.25/share (a 31% premium); 15.78x sales |
|
February |
Pfizer/Hospira (injectable generics) |
$17.1B: $15.4B in cash, $1.75B in debt; 3.44x sales |
|
February |
Valeant/Salix (gastrointestinal-focused specialty pharma) |
$11B: $173/share (a 17% premium); 8.95x sales |
|
January |
Shire/NPS Pharmaceuticals (gastrointestinal and orphan drugs) |
$4.9B: $46/share (a 19% premium); 31.66x sales |
|
March |
Teva/Auspex (rare movement disorders) |
$3.2B: $101/share (a 32% premium) |
Strategic Transactions
In the highest M&A of the quarter, AbbVie paid close to $20 billion in cash and stock for cancer drugmaker Pharmacyclics Inc. [See Deal] (See (Also see "AbbVie Banks On “Pipeline In A Molecule” Strategy For Imbruvica" - Pink Sheet, 9 Mar, 2015.).) Anxious to get its hands on Pharmacyclics’ Bruton’s tyrosine kinase inhibitor Imbruvica (ibrutinib), AbbVie reportedly outbid two other rival pharmacos (including Johnson & Johnson, with which Pharmacyclics has a 2011 partnership for Imbruvica [See Deal]). FDA approved since November 2013 for mantle cell lymphoma, Imbruvica had 2014 US sales of $492 million, and those sales are expected to increase to $1 billion for this year with approval in expanded cancer indications. The addition of Pharmacyclics will help AbbVie build a hematologic cancer portfolio and reduce the company’s dependence on Humira (adalimumab) – the blockbuster autoimmune drug that makes up about 60% of AbbVie’s product sales – which loses US patent exclusivity next year. (See (Also see "Humira’s Strong Growth Is A Double-Edged Sword For AbbVie" - Pink Sheet, 23 Apr, 2015.).)
Pfizer bought sterile injectable generics maker Hospira Inc. [See Deal] (see (Also see "Pfizer Makes Its M&A Move; Hospira Will Be More Of A Bolt-On, Read Says" - Pink Sheet, 5 Feb, 2015.)) to strengthen its Global Established Pharmaceutical business (made up of mature brands and drugs that have lost exclusivity) and broaden its biosimilars portfolio with Hospira’s injectables offerings, including Inflectra (infliximab), a copy of J&J’s rheumatoid arthritis mAb Remicade, launched in Europe in 2013 with partner Celltrion Inc. [See Deal]
Pfizer wasn’t the only one willing to pay big to acquire a generics player: Concordia Healthcare Corp. bought Covis Pharma SARL for $1.2 billion in cash [See Deal] – over eight times the private company’s projected 2014 revenues – gaining the Swiss spec pharma’s portfolio of 18 marketed generics across various therapeutic areas such as cancer, cardiology, and infectious diseases. Also, Allergan PLC paid $460 million for private generics company Auden McKenzie Ltd.[See Deal] and its 175 generic and branded products as well as 40 pipeline candidates, making Actavis the third-largest UK pharmaceutical supplier. (During Q1 Actavis also divested its branded respiratory portfolio to AstraZeneca [See Deal], which paid $700 million for US and Canadian rights to this franchise, and sold its Aptalis Pharmatech Inc. pharmaceutical outsourcing and R&D services business to TPG [See Deal].)
In March, Valeant Pharmaceuticals won the bidding war for Salix Pharmaceuticals Ltd. [See Deal] with its $173 per share cash offer – up from the $158 it had originally proposed in February – beating out competing suitor Endo International PLC, which in March launched an unsolicited $171.81/share cash and stock bid for Salix that it later withdrew. [See Deal] (See (Also see "Valeant’s Higher Bid Seals The Deal With Salix, Ending Endo’s Quest" - Pink Sheet, 16 Mar, 2015.).) For Valeant, the deal adds Salix’s mostly gastrointestinal disease therapeutics, particularly Xifaxan (rifamixin) for hepatic encephalopathy and travelers' diarrhea (it’s also awaiting FDA approval in an irritable bowel syndrome with diarrhea indication, for which BioMedTracker predicts a 93% likelihood of approval) and Relistor (subcutaneous methylnaltrexone bromide for opioid-induced constipation), for which Valeant hopes to develop an oral version.
Also getting into the GI space was Shire PLC through its buy of NPS Pharmaceuticals Inc. [See Deal] NPS adds to Shire’s offerings the FDA-approved Gattex (teduglutide) for short bowel syndrome; Natpara/Natpar (recombinant parathyroid hormone) under review in the US/Europe, respectively, for hypoparathyroidism (upon approval it would be the first available treatment); and NPSP795, a calcium-sensing receptor antagonist in Phase IIa for adult autosomal dominant hypocalcemia. During Q1 Shire also exercised an option to acquire Meritage Pharma Inc.![]() for $70 million up front and up to $175 million in potential development and regulatory earn-outs, getting hold of the rare disease player’s in-licensed pipeline of six GI candidates, including its Phase III-ready lead compound, oral budesonide suspension (OBS) for eosinophilic esophagitis. Shire got the option as part of its 2013 buy of Viropharma [See Deal], which in 2011 paid Meritage $7.5 million up front (and $12.5 million in potential earn-outs) for the option to buy the company for as much as $69.9 million more (plus additional earn-outs). [See Deal]
for $70 million up front and up to $175 million in potential development and regulatory earn-outs, getting hold of the rare disease player’s in-licensed pipeline of six GI candidates, including its Phase III-ready lead compound, oral budesonide suspension (OBS) for eosinophilic esophagitis. Shire got the option as part of its 2013 buy of Viropharma [See Deal], which in 2011 paid Meritage $7.5 million up front (and $12.5 million in potential earn-outs) for the option to buy the company for as much as $69.9 million more (plus additional earn-outs). [See Deal]
Teva Pharmaceutical Industries Ltd.also gained a rare disease player in its March buy of Auspex Pharmaceuticals Inc.![]() , which is focused on movement disorders. [See Deal] Teva recently announced a strategy to refocus efforts toward neurological disease, and with this transaction is getting Auspex’s Phase III program in Huntington's chorea, SD809 (with a proposed brand name of Austedo; deutetrabenzine). After meeting the primary and secondary endpoints in top-line trial data, BioMedTracker increased SD809's likelihood of approval by 7% to 59%. An NDA is planned for mid-2015, and Teva says forecasted sales five years from launch could reach $2 billion.
, which is focused on movement disorders. [See Deal] Teva recently announced a strategy to refocus efforts toward neurological disease, and with this transaction is getting Auspex’s Phase III program in Huntington's chorea, SD809 (with a proposed brand name of Austedo; deutetrabenzine). After meeting the primary and secondary endpoints in top-line trial data, BioMedTracker increased SD809's likelihood of approval by 7% to 59%. An NDA is planned for mid-2015, and Teva says forecasted sales five years from launch could reach $2 billion.
In February Bristol-Myers Squibb Co. paid $1.25 billion ($800 million up front and another $450 million in earn-outs) to buy cancer start-up Flexus Biosciences Inc. [See Deal], a transaction which, along with the aforementioned Concordia/Covis deal, made up most of the $2.5 billion total spent on acquiring start-ups during Q1.
Alliances
Biopharmas began 2015 partnering at an active pace. Franchise divestments were significantly represented in the pack of larger deals – or those 16 that were worth $100 million or more. The top money maker of Q1, Depomed, bought US rights to Janssen's chronic pain drug Nucynta (tapentadol) and related assets for $1.05 billion. (See Exhibit 5.) The Janssen division divested Nucynta, which originally came from Grunenthal GMBH [See Deal], to focus on higher-priority drugs. Depomed says it plans to relaunch Nucynta with full support of its sales force. (See (Also see "Depomed To Make Treasure Out of J&J Trash With Nucynta Buy" - Pink Sheet, 16 Jan, 2015.).)
Actavis reduced its investment in respiratory diseases by selling its US and Canadian branded portfolio in that area to AstraZeneca for $600 million up front. On the contrary, AZ has put a lot of resources behind its respiratory offerings, which booked sales of $4.6 billion in 2014 thanks to leading product Symbicort (budesonide/formoterol). In July 2014 the Big Pharma also bought the respiratory business of Almirall SA for $875 million. [See Deal] In the Actavis deal, AZ gets the marketed COPD products Tudorza Pressair (aclidinium) and Daliresp (roflumilast), which together pulled in $230 million in sales, and the fixed-dose Phase III combination LAS40464 (aclidinium/formoterol), also for COPD. (See (Also see "AstraZeneca Capitalizing On Respiratory With Actavis’ Divestiture" - Pink Sheet, 5 Feb, 2015.).) Actavis, which got these assets when it bought Forest last year [See Deal], will instead focus on core areas including neurology, women’s health, urology, GI, anti-infectives, cardiovascular, dermatology, and ophthalmology.
AZ was actually the most active in-licenser during Q1. Combined with its MedImmune LLC division, the company signed five deals focused in various areas; aside from respiratory, they included CRISPR research (Thermo Fisher Scientific Inc.[See Deal]), metabolic diseases (Joslin Diabetes Center[See Deal]), cancer (Omnis Pharmaceuticals Inc.[See Deal]), and autoimmune disorders (Orca Pharmaceuticals Ltd.)[See Deal]
Exhibit 5
Top Biopharma Alliances, Q1 2015
|
Month |
Licensee/Licenser |
Subject(s) of Deal |
Potential Deal Value*; Terms |
|
January |
Depomed/Janssen |
Nucynta (tapentadol) for pain, including extended- and immediate-release formulations |
$1.05B; Depomed will fulfill royalty obligations owed to Grunenthal |
|
March |
Novartis/Aduro |
Preclinical cyclic dinucleotide (CDN) program in immuno-oncology |
$750M; $225M UF ($200M cash and $25M for 2.7% stake), $250M in dev. MS, $250M in reg. MS, and another $25M investment |
|
February |
AstraZeneca/Actavis |
Actavis' branded respiratory drug portfolio in the US and Canada; including Tudorza Pressair (aclidinium) and Daliresp (roflumilast), both for COPD, and Phase III LAS40464 (aclidinium/formoterol) for COPD |
$700M; $600M UF, $100M in undisclosed contractual consents and approvals, and low-single-digit royalties |
|
January |
Valeant/Dendreon |
Dendreon's assets including Provenge (sipuleucel-T) for advanced prostate cancer |
$495M |
|
March |
Bristol-Myers Squibb/Bavarian Nordic |
Option on Phase III Prostvac (rilimogene galvacirepvec) for asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer |
$480M; $60M UF, $80M option exercise fee, $50M–$230M in MS tied to Phase II results, $110M in reg. MS, $495M in sales MS, and tiered double-digit royalties |
*Note: Potential Deal Value is the sum of up-front fees/equity plus pre-commercialization money.
Strategic Transactions
The franchise divestment group also featured purchases of more troubled products. One of those was Dendreon Corp.'s Provenge (sipuleucel-T), a once heralded breakthrough cancer vaccine that was projected to reach over $4 billion in annual sales by 2020, but instead never achieved its full potential due to complicated reimbursement issues and competition. In 2014, four years post-approval, the drug's sales only totaled $224 million. Valeant Pharmaceuticals acquired the assets of Dendreon, which had filed for Chapter 11 in November 2014, for $495 million. (See (Also see "Valeant To Buy Dendreon’s Never-Was Cancer Vaccine Provenge" - Pink Sheet, 30 Jan, 2015.).) (Valeant had a busy Q1; in addition to buying Provenge, Salix Pharmaceuticals, and raising $1.43 billion in debt, Valeant also acquired several hospital products from Marathon Pharmaceuticals LLC. [See Deal])
Zogenix Inc.’s severe pain reliever Zohydro ER (hydrocodone), another troubled drug, found a buyer in Pernix Therapeutics Holdings Inc., which paid $100 million up front and promised up to $284 million in milestones. [See Deal] In 2013, Zohydro became the first pure extended-release hydrocodone drug ever approved; however the FDA came under fire for clearing the medication, which doesn't have abuse-deterrent properties. Just before Zogenix divested Zohydro, it gained approval for a BeadTek formulation of the drug that it designed to be tamper-resistant. This reformulation is part of the Pernix deal, as is a similar abuse-deterrent tablet in development with Altus Formulation Inc. [See Deal]
Transactions for marketed products such as these represented the largest volume of alliances in the first quarter, followed by early-stage discovery and preclinical candidates. (See Exhibit 6.)
Exhibit 6
Q1 2015 Alliances By Phase
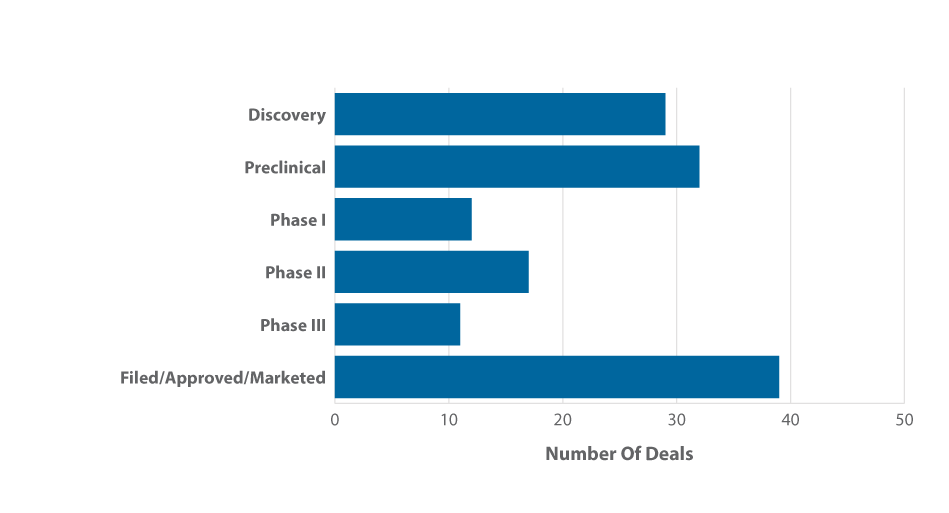
Note: Deals involving more than one phase were counted more than once (for each phase represented).
Strategic Transactions
Immuno-oncology dealmaking continued at an active pace in the start of 2015, and there were several stand-outs among the first quarter's bigger deals. Novartis AG formed an immuno-oncology research group that among other initiatives will help drive a new multi-year alliance between Novartis Institutes for BioMedical Research Inc. (NIBR) and Aduro Biotech Inc. [See Deal] For $225 million up front (including a 2.7% equity stake in Aduro), NIBR gets ex-US rights to Aduro's cyclic dinucleotides, which activate the Stimulator of Interferon Genes (STING) pathway. (See (Also see "Novartis Aims To Leap-Frog Into Next Immuno-Oncology Wave With Aduro" - Pink Sheet, 1 Apr, 2015.).) In Q1, Novartis also completed its acquisition of GlaxoSmithKline PLC's oncology business. [See Deal] To make that transaction happen, Novartis Pharma AG divested the Phase III BRAF inhibitor encorafenib to its partner Array BioPharma Inc. [See Deal] The European Commission is also requiring GSK and Novartis to sell off other assets to clear their consumer [See Deal] and vaccines [See Deal] transactions announced in April 2014. [See Deal]
Other immuno-oncology highlights from the first quarter included Bristol-Myers Squibb's exclusive global license to Rigel Pharmaceuticals Inc.'s preclinical transforming growth factor (TGF) beta receptor kinase inhibitors, which may be combined with BMS' melanoma immune checkpoint inhibitors Opdivo (nivolumab) and Yervoy (ipilimumab) [See Deal]; and a pair of alliances by Intrexon Corp. In January 2015, Intrexon, along with Ziopharm Oncology Inc., got rights to the University of Texas’ MD Anderson Cancer Center’s chimeric antigen receptor T (CART) cell technologies, plus a sublicense to the Sleeping Beauty nonviral DNA plasmid-based gene transfer system from the University of Minnesota. [See Deal] The universities received $100 million in Intrexon and Ziopharm shares and $15 million to $20 million annually in R&D funding over three years. Two months later, Intrexon and Merck KGAA’s Merck Serono SA agreed to work on CART therapy improvements, building off the licensed technologies from the universities, in an agreement worth $115 million up front and up to $826 million in development, regulatory, and sales milestones. [See Deal]
In one of the largest up-front payments paid in recent years for a gastrointestinal medicine, AstraZeneca granted Daiichi Sankyo Co. Ltd.'s Daiichi Sankyo Inc. US co-promotion rights to its approved opioid-induced constipation therapy Movantik (naloxegol) for $200 million. Launch is expected in April 2015, and sales are forecast to reach $2.9 billion worldwide by 2020. The deal could expand Daiichi's US presence and help build out a broader pain management portfolio. (See (Also see "AZ Alliance Bolsters Daiichi’s US Primary Care Presence" - Scrip, 19 Mar, 2015.).) In Q1, GI deals, including AZ/Daiichi's, represented 9% of the total and were among the top three partnered therapeutic areas. (See Exhibit 7.)
Exhibit 7
GI Joins Cancer, Neurology Among Highly Partnered Therapeutic Areas, Q1 2015
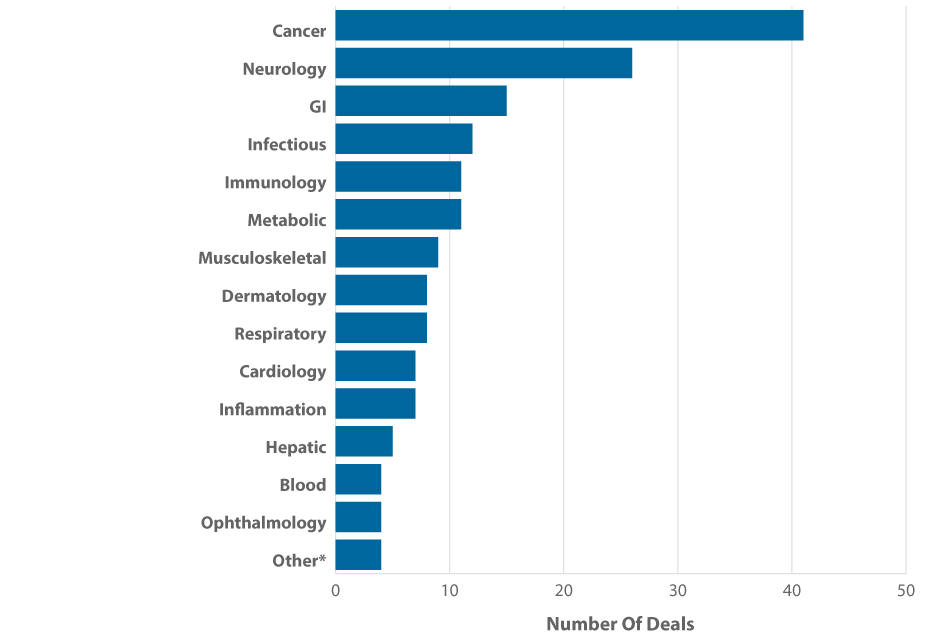
*The Other category represents four deals, one each in Dental, Gynecology, Poison (Antidote), and Wound Healing.
Note: Deals involving more than one therapeutic area were counted more than once (for each TA represented).
Strategic Transactions