Cartilage Regeneration: Solving The Unsolvable?
Executive Summary
CartiHeal's simple, cell-free construct may offer a potential solution to one of the greatest unsolved problems in musculoskeletal surgery – cartilage regeneration. An interview with Nir Altschuler, founder and CEO of the Israeli start-up.
- The search is on for technologies that fill the gap in treatment options for patients with knee pain.
- Technologies for regenerating articular cartilage have fallen short, producing a scar tissue called fibrocartilage rather than true hyaline cartilage.
- Israeli start-up CartiHeal has developed an off-the-shelf cell-free construct derived from coral that it says reproducibly regenerates the ”real deal” — type II hyaline cartilage.
With joint replacement the only game in town for treating worn out arthritic knees, the search is on for technologies that can be used earlier in the treatment continuum and stave off the progression of degenerative joint disease. Last year, close to 800,000 people in the US had their knees replaced with a total joint and this number is expected to increase to over 900,000 by 2019. (See Exhibit 1.) Increasingly, total knees are implanted in younger patients who no longer want to live with stiff and painful joints but have no other alternative.
Treating knee pain is big business. Last year in the US, $4.5 billion was spent on knee replacement implants and nearly $1 billion on hyaluronic acid injections and this does not include the billions of dollars pharmaceutical companies reap on selling non-steroidals and other pain relieving drugs.
Exhibit 1
US Knee Replacement Procedure Volumes, 2014–2019E
|
|
2014 |
2015E |
2016E |
2017E |
2018E |
2019E |
CAGR |
|
Number of Procedures |
|
|
|
|
|
|
|
|
Primary Total Knee |
611,805
|
631,383 |
652,218 |
673,089 |
693,955 |
715,468 |
3.2% |
|
Revision Knee |
79,275 |
84,428 |
89,916 |
95,580 |
101,506 |
108,003 |
6.4% |
|
Partial Knee |
66,046 |
68,886 |
71,849 |
74,938 |
78,160 |
81,521 |
4.3% |
|
Patello-Femoral Joints |
15,220 |
15,410 |
15,603 |
15,798 |
15,995 |
16,195 |
1.3% |
|
Total Knee Replacements |
772,346 |
800,107 |
829,586 |
859,406 |
889,617 |
921,187 |
3.6% |
|
YoY Growth Rates |
3.6% |
3.6% |
3.7% |
3.6% |
3.5% |
3.5% |
|
SOURCE: BioMedGPS’ SmartTRAK Business Intelligence
The culprit is knee osteoarthritis, a natural function of aging where craters form in the joint’s articular surface as a result of overuse. It may also develop at a young age following a traumatic injury to the articular cartilage often from sports, a condition known as post-traumatic arthritis. Besides a total joint, the only surgical options available, for the most part, are allografts, microfracture, and a new procedure called subchondroplasty. Allografts such as osteochondral grafts are not widely used; they are expensive, limited by supply, and pose a risk of disease transmission.
Microfracture involves poking holes in the cortical bone underneath the damaged cartilage to release cells and marrow elements with the goal of filling the defect with a type of scar tissue called fibrocartilage. The pros of microfracture are that it is cheap, can be done arthroscopically, and doesn’t require special instrumentation; the cons are that an inferior tissue is regenerated that does not possess the durability of normal hyaline cartilage.
Subchondroplasty, on the other hand, treats bruises in the subchondral bone – believed to be a major cause of knee pain – by stabilizing it with a toothpaste-like material that hardens in situ. Zimmer Biomet Holdings Inc. was the first to jump into the early intervention game by acquiring both Knee Creations, the developer of subchondroplasty, in 2013 and Etex, the manufacturer of the bone replacement material, last year. [See Deal]
However, none of these procedures address the underlying cause of knee degeneration – disruption of the articular cartilage, the slick white glistening tissue covering the surface of joints. Once believed to be irreparable owing to its poor propensity to heal, attempts at regenerating articular cartilage have fallen short with most technologies producing fibrocartilage, which is “hyaline like” and composed of type I collagen, rather than the real deal – “true” type II hyaline cartilage. Cartilage regeneration has been the subject of intense research for decades and researchers have tried a medley of expensive cell-based and biotech approaches (e.g., stem cells, genes, growth factors, chondrocytes from the patient or a donor) delivered on or in combination with almost every type of scaffold in order to coax the body to regenerate hyaline cartilage.
In the US, orthopedic manufacturers such as Zimmer and Arthrex Inc., along with allograft suppliers (RTI Surgical Inc., AlloSource, LifeNet Health Inc., Musculoskeletal Transplant Foundation), have been marketing a variety of human tissue-derived cartilage grafts that essentially bypass FDA device regulations. These range from fresh osteochondral grafts to cell-based products such as Zimmer’s De Novo NT juvenile chondrocytes acquired from Isto Technologies Inc., and Osiris Therapeutics Inc.’s Cartiform; Osiris entered into a distribution agreement with Arthrex late last year. [See Deal] While there are a handful of products in Phase III trials, to date, only one cartilage repair technology, Vericel Corp.'s Carticel, acquired from Sanofi/Genzyme Corp. last year [See Deal], has made its way through the FDA approval process, and that was almost 20 years ago.
BioMedGPS projects the US market for cartilage replacement will reach almost $97 million in 2015, with cell-based constructs, including Vericel’s Carticel and Zimmer’s DeNovo NT, accounting for over half of total revenues, followed by osteochondral grafts and human tissue or allogenic-derived implants – the latter considered the fastest growing market segment. (See Exhibit 2.)
Exhibit 2
US Cartilage Replacement Market By Segment, 2014–2019 ($M)
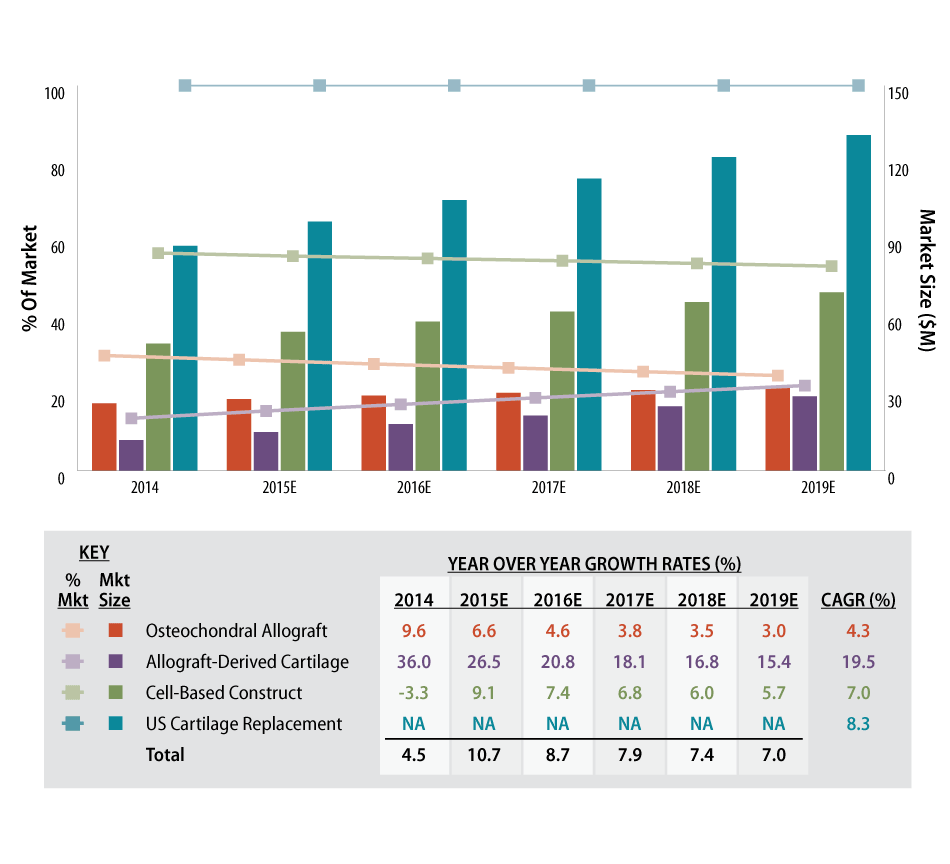
SOURCE: BioMedGPS’ SmartTRAK Business Intelligence
In May, researchers from around the world met in Chicago at the12th World Congress of the International Cartilage Research Society (ICRS), a think-tank of experts in the field of cartilage regeneration. Among the promising technologies presented at the ICRS meeting was a simple, off-the shelf, cell-free construct developed by CartiHeal Ltd., an Israeli start-up, which received Israel’s Ministry of Economy's prestigious “Excellence Award – Outstanding Company” in 2013. CartiHeal stands out from the pack owing to the robust preclinical and clinical research presented at the ICRS meeting demonstrating regeneration of true hyaline cartilage and its underlying subchondral bone. IN VIVO interviewed Nir Altschuler, CartiHeal’s founder and CEO, regarding how he may have discovered a solution to one of the most elusive problems in musculoskeletal surgery.
IN VIVO: Tell us about CartiHeal and how it was formed. Where did you get your initial funding and how much was it?
Nir Altschuler: After getting my biotech engineering and MBA degrees, I started working as VP of business development at Peregrine Ventures, an Israeli venture capital firm focused on investing in early-stage medical technologies where one of my responsibilities was to scout for new technologies for the firm to invest in. One day, I came across an interesting study that was performed 10 years before by one of my former professors from the Department of Biotech Engineering at Ben-Gurion University and invited him to show his research to the VC firm. In that study, a coralline scaffold was implanted into an osteochondral defect in a rabbit femoral condyle that demonstrated regeneration of bone and fibrocartilage.
While he was presenting his research, I thought that there must be a way to improve the coral so it can regenerate hyaline cartilage and not fibrocartilage and eventually to develop a commercial product that can be used to treat cartilage defects, a huge unmet market.
Following that meeting, and in parallel with my work at the VC firm, I spent the next few months researching potential ways for transforming coral into a cartilage regenerative scaffold. Once I felt that I had established a solid hypothesis, I wrote the patent applications and started looking for money under the support of the university. Naturally, I approached Peregrine Ventures first, who loved the concept and provided the initial funding. One year after that meeting, CartiHeal was formed in July 2009.
Peregrine and a group of Swiss investors, Pertec Management, provided the first $200,000 of funding alongside government support in the amount of $500,000. These funds were used to establish the company, perform basic research, optimize the technology, and complete a six-month feasibility study in goats.

Nir Altshchuler
Coral has been used as a bone replacement material. What makes this material unique for cartilage regeneration? Can you describe the structure of the implant as well as its mechanism of action? Where does CartiHeal stand regarding its IP?
Coral has a very similar structure to human bone in terms of mechanical strength and interconnected porosity allowing it to function as an osteoconductive and osteotransductive scaffold. When placed in a boney defect, coral remodels and forms new bone. It has been used since the ’70s as a bone replacement material, either in its native state, in the aragonite crystalline form, or after conversion to hydroxyapatite. CartiHeal’s uniqueness is that we’ve developed a proprietary biphasic coralline-based scaffold, Agili-C, capable of inducing simultaneous bone formation in the bone phase and cartilage formation in the chondral phase.
We have spent a lot of time and effort in order to understand the mechanism of action leading to hyaline cartilage formation with the Agili-C. At the chondral phase, the Agili-C stimulates chondrocytes from the surrounding cartilage to migrate into the scaffold depositing an extracelluar matrix while attracting stem cells from the bone marrow to differentiate into chondrocytes. These two processes occur simultaneously and lead to hyaline cartilage formation. This unique mechanism of action was confirmed in intensive studies by leading laboratories around the world.
The bone phase of the implant is composed of aragonite, whereas the cartilage phase has a modified 3-D structure composed of an aragonite-hyalurante composite that stimulates cartilage formation.
This technology is protected by granted patents around the world, including five patents in the US.
The Agili-C implant contains hyaluronic acid. Where did the concept of incorporating hyaluronic acid come from?
I came across a study on stem cells seeded on barnacles with and without the presence of hyaluronic acid. The hyaluronic acid increased the chondrogenic capability so the stem cells differentiated into chondrocytes. I thought if it worked on a barnacle, it might work on coral. So we combined the coral with the hyaluronic acid to create the biphasic implant with coral comprising the bone phase and modified coral and hyaluronic acid at the cartilage phase to stimulate stem cell proliferation and differentiation.
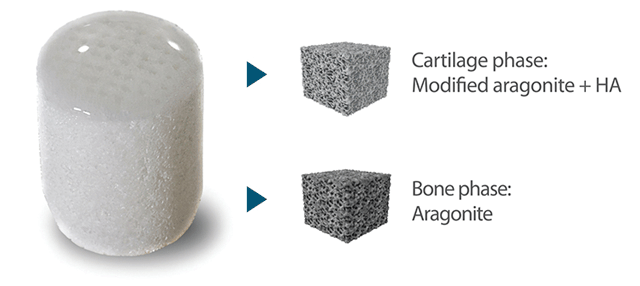
Agili-C Two Phases
Tell us about the goat study; what did the results show?
Since we knew we had enough funding for only one long-term study, we designed different prototypes using different combinations of technologies based on the coral modifications and patented all of them. Then we brought some leading orthopedic surgeons with expertise in cartilage regeneration, Andrew Levy and Ken Zaslav from the US and Elizaveta Kon from Italy [all MDs], to Israel to operate on the goats using the different prototypes. The results of the goat studies were dramatic and showed that defects repaired with the Agili-C were healed and filled with hyaline cartilage as confirmed by the presence of proteoglycans and collagen type II and absence of collagen type I. The repaired tissue was well integrated into the adjacent cartilage and subchondral bone, which was also fully reconstructed. The histological results were confirmed by NAMSA in a GLP evaluation. At that moment in time, we knew we had something unique and that perhaps we could succeed in a field where so many before us have failed.
How were you able to recruit three such reputable surgeons from different parts of the world to come to Israel to operate on goats when you had no relationships with them in the past?
During the year before CartiHeal was formed, there was an orthopedic meeting in Israel and one of the keynote speakers, Andrew Levy, an orthopedic surgeon from New Jersey, was discussing scaffolds for cartilage repair. After the talk, I approached Dr. Levy and invited him for a beer over which I shared my idea for using converted coral as a scaffold for cartilage regeneration. After asking challenging questions, he agreed to meet again the next day to discuss the concept in depth. The next day, we dived into the basic science and at the end our meeting, he was convinced that there was a chance “it might work.” Andrew introduced me to Ken Zaslav and Elizaveta Kon, who liked the concept and agreed to join us as consultants before the company was officially funded.
So you had promising results from the goat study but were running out of money; what did you do then?
It’s a good story. I was meeting with Michael Tal [MD] , a radiologist and the Managing Director of Access Medical Ventures, a newly formed venture firm, regarding another technology I was working on that involved RF treatment for cancer. After showing him the technology, there was 15 minutes left in the meeting, so he asked me if I had anything else I wanted him to see. We had just received the results from the first pilot preclinical study on the Agili-C with hyaline cartilage formation, so I reviewed the histology with him. The next day I got a term sheet and a week later, signed the agreement for a $500,000 investment in CartiHeal.
What did you do with the additional funding?
We completed a second goat study, obtained a CE mark, and performed first-in-man implants – and all for under $1.2 million in less than two years! After that, we raised another $10 million round from Accelmed and Elron, two of the most active and successful venture firms in Israel.
Tell us about your clinical experience with the Agili-C so far. How many implants have been done to date and for what indications?
We just presented this data at the ICRS. Altogether, over 130 patients have been treated with Agili-C implants. Of these, approximately 20% of the cases were done with a concomitant ACL reconstruction and/or menisectomy and 50% of the patients had some arthritic changes. We have 18-month follow-ups on most of these patients with 50 of them at the 24-month mark.
As we gained more confidence in the technology, the surgeons who were using the Agili-C started to push us from simple focal defects toward more challenging cases like multiple lesions, larger cartilage defects, osteochondral defects, and post-traumatic osteoarthritis, as well as lesions in other joints such as the foot and ankle. In May 2014, we expanded the CE mark and increased the size of the implants to 20 mm in diameter. To date, over 50 patients have received the larger implants for bigger and deeper osteochondral defects as well as for multiple defects. Up to three of the larger implants have been implanted in a single knee essentially covering the entire joint’s load-bearing surface. This is a huge population of patients that essentially have no other treatment options
Who is the ideal patient for the Agili-C?
Generally speaking, we are targeting three patient populations today: focal traumatic chondral lesions, osteochondral defects, and post-traumatic OA. In the continuum of care, the Agili-C can capture most of the huge unmet need of patients who have failed conservative treatment (e.g., physiotherapy, NSAIDs, and hyaluronic acid injections) or are either too young or not a candidate for knee replacement. (See Exhibit 3.)
Exhibit 3
Osteoarthritis Continuum Of Care
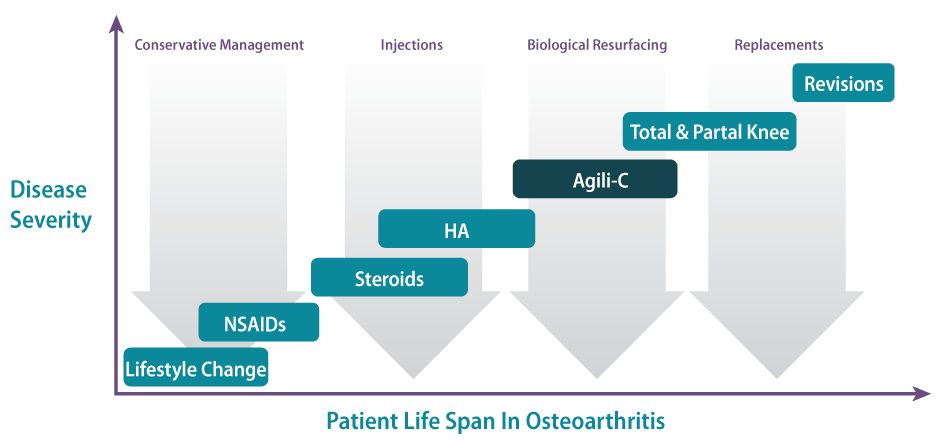
SOURCE: CartiHeal
What is your longest follow-up? How do the long-term results look so far?
Our longest follow-up is four years. The first implantation was done in June 2011 on a 47-year-old male with a Grade 4 ICRS lesion on the load-bearing region of the medial femoral condyle. At six months post-implantation, he was skiing and after 12 months, he completed a 180-km cycling marathon. He remains active and very satisfied.
We recently did an interim analysis and the results are very promising. Knee function scores such as IKDC, KOOS, which includes pain, symptoms, activities of daily living, sports activities, and quality of life, and Lyshom are showing improvements at all time points relative to baseline as early as three-months post-implantation with a continuation of improvement over time.
Most of the patients report nearly immediate pain relief following surgery. We think this is because the diseased and inflamed subchondral bone under the chondral defect – which is a known pain generator – is removed and replaced with a stable construct that allows tissue ingrowth and then remodels into bone and cartilage.
Interestingly, on MRI at three months post-implantation, over 70% of the defect is filled with cartilage and this improves with time to over 90% at 24 months.
How easy or reproducible is the implantation technique? How long does the procedure take? Is it arthroscopic? Does it require special tools or training?
The procedure is very simple, intuitive, and fast. The implantation itself takes about three minutes and there is virtually no learning curve, and it is done solely with the Agili-Kit, a surgical tool set that was designed for proper implantation of the implant. The size of the implants ranges from 10 mm to 20 mm in diameter, so the procedure is done through a small arthrotomy.
There were some very interesting findings presented at ICRS regarding “red” or “white” coral. How did you discover this and what is the clinical significance? How can you be certain that the Agili-C implant comprises only “red” coral?
In the initial 12-patient pilot study, most of the patients had very good outcomes with excellent cartilage and subchondral bone reconstruction, but there were also patients in which the implant – for some unexplained reason – did not integrate and had to be removed. As a young start-up, we faced a dilemma, we either shut down the company or find an answer to the problem. Fortunately, we had a very supportive team of clinicians and solid investors who believed in us as well as the technology and provided an additional $5 million in funding specifically to research the cause of the unexplained failures.
So we formed two teams to search for answers: one group looked for a mechanical solution while the other searched for a biological one. Six months was spent searching for an answer. Every evening before leaving the office, I would look at the videos and images of the surgeries searching for clues. Then one day I realized that all of the implants that were removed didn’t absorb any of the patients’ blood and remained “white” in color while the implants that integrated and remolded absorbed blood, and were red in color. Finally we deciphered this enigma. We call it the “red-white phenomenon” – “red” are corals that absorb blood while “white” are corals that do not. We conducted several studies and confirmed that this was the reason for the lack of integration observed in some Agili-C patients.
This discovery led to intensive development of a proprietary and rigorous manufacturing and quality control process that ensures each and every Agili-C implant is characterized by optimal blood absorption capabilities. We believe this is absolutely essential for the regeneration of both cartilage and bone. Since then, all patients have received “red” Agili-C implants and the results have been excellent with hyaline cartilage and subchondral bone formation.
Where do you harvest the coral from? Are there any issues with supply?
Theoretically, there is no issue with supply; however, global trade on coral is very strict and each piece of coral that is imported must be certified by CITES, a global organization that regulates the trade in fauna and flora. In addition, the geographical area itself and the trader must also be certified. We are harvesting corals from different locations and all of them are certified by CITES.
Since only a small amount of material is needed to produce an implant, we can obtain thousands of implants from 1 cubic meter of coral.
How does the Agili-C differ from other cartilage replacement technologies?
That's simple – unlike other technologies, it regenerates true hyaline cartilage. The Agili-C is a simple, cost-effective, off-the-shelf, cell-free device that can be implanted in a single-stage procedure. Other technologies, like autologous chondrocyte implantation or ACI, require two operations to: (1) harvest cells and; (2) reimplant them back into the patient. Plus, these technologies are expensive and outcomes are inconsistent.
We have seen bone and hyaline cartilage formation on imaging (i.e., MRI, X-ray, and CT) and have 12-month histological results from NAMSA that confirm the presence of hyaline cartilage and complete regeneration of the subchondral bone. In addition, there is pain relief, symptom relief, high satisfaction rates, and an easy and reproducible implantation technique.
What is your regulatory strategy for the US and OUS? Have you met with the FDA? When do you expect to begin US trials?
Currently, a CE mark was issued in June 2011 for the small-sized implants. In May 2014, we received an expanded CE mark for the larger implants for the treatment of cartilage and osteochondral defects, not limited to a specific joint or clinical condition. We are planning to initiate commercialization with a well-targeted group of surgeons and hospitals in the EU in late 2016. Our plan is to start a dialogue with the FDA in the next few months with the goal of starting a pivotal study in the US next year.
Only one cartilage replacement product has been approved on the US market and that was almost two decades ago. Why do you think the FDA has not approved any other technology?
The FDA has been very wary of cartilage repair technologies, possibly, because most of them regenerate only fibrocartilage and do not demonstrate sustainable superiority over microfracture. Also, the guidelines issued by the FDA required very expensive and lengthy IDE studies and, frankly, some companies have simply walked away although this is starting to change. So far, we have evidence of cartilage and bone regeneration in a significant number of patients receiving the Agili-C, with significant improvements in pain and quality of life reported. We believe that we will have strong and robust long-term clinical data that will support Agili-C's approval in the US.
You have had a CE mark for four years, but have not chosen to commercialize – why?
We obtained a CE mark for the smaller-size implant [6–10 mm in diameter] in 2011. Since our goal is to treat large defects and post-traumatic OA, we used that CE mark to conduct a series of post-marketing clinical studies to support our request to increase the size of the implant. In May 2014, our notified body approved our request allowing us to use up to 20 mm in diameter implants. Since then we are treating more severe cases. Our strategy is to gather a significant amount of clinical data in a variety of indications and with long-term follow-up before starting commercialization.
You must have very patient investors; what is the hold up in not going to the FDA or starting to commercialize your product?
All of our investors were former founders and CEOs of medical device companies, so besides funding they bring a great deal of professional experience. They understand that strong and excellent clinical data are the keystones for building a strong and sustainable company. Even though the results with small defects look really good, the possibility of treating more severe cases, like post-traumatic osteoarthritis, opens up a whole new market for us with tremendous potential that will maximize the value of the company.
Recently, other cartilage regeneration companies such as Histogenics have gone public; is this something you are considering or will consider in the future?
This is an option we are definitely considering.
In the US, we have seen a number of companies [Arthrex, Osiris, ISTO, Zimmer] marketing allogenic-derived cartilage implants comprising human tissue and exempt from device regulations. How will you compete with these technologies?
Our lead product, Agili-C, is an off-the-shelf cartilage regenerative implant with a three-year shelf life, that has demonstrated the ability to regenerate hyaline cartilage and subchondral bone in a significant number of patients and a variety of clinical conditions. Although in the same space, the indication we are targeting is much broader than some of the technologies you mentioned, so the overlap is potentially small.
How big do you see the market for Agili-C?
We believe there is a tremendous market for the Agili-C. We estimate that the market for treating traumatic and early-stage degenerative lesions of the knee, ankle, and great toe is a multibillion-dollar opportunity worldwide. We believe that the Agili-C will become the gold standard for treating a cartilage defect over the next few years.
Sharon O'Reilly ([email protected]![]() ) is founder, president, and chief executive officer of BioMedGPS LLC, and was the founder of Medtech Insight.
) is founder, president, and chief executive officer of BioMedGPS LLC, and was the founder of Medtech Insight.