TG Therapeutics Builds A Business Model For Today
Executive Summary
The speed with which TG Therapeutics burst on the scene, along with the impressive potency and safety of its novel combinations of cancer drugs, has perhaps blinded observers to the unique business model that has carried it this far.
- TG has devised a business model that is designed to confer multiple benefits across its value chain. It also points the way for small-cap cancer companies in the age of immuno-oncology: start with proprietary combinations of validated mechanisms, layering on riskier assets over time.
- TG’s programs – in oncology and in the soon-to-be-christened autoimmune franchise – are largely based on a B-cell depleting backbone combo. If its unprecedented safety profile fades in Phase III trials, the company could be at risk.
- Two pivotal trials give TG a good head start, but it will need to stay ahead of the pack, continuing to license in superior assets and assembling them in novel combinations.
Cancer specialist TG Therapeutics Inc. has recently shown positive mid-stage data for its assets, both alone and in combination, in patients with advanced B-cell cancers. The speed with which it burst on the scene, combined with the potency and safety of its drugs, has perhaps blinded observers to the unique business model that has carried it this far.
The main element in the model is TG’s dedication from the start to proprietary combinations of its own drugs. TG understood that owning its pipeline would satisfy several key challenges facing biopharmas in the age of combination therapy.
It would allow the company to control the design and conduct of complex trials involving combinations or sequencing of two or more drugs. It would obviate the need to negotiate involved IP and logistical arrangements with big pharma. TG would be able to keep the lion’s share of revenue should its drugs reach market, and it would give TG sole discretion over pricing. That last benefit addresses the pressure that companies, increasingly cancer companies, come under to price their drugs responsibly. Moreover, it flies in the face of current wisdom that says combinations of cancer drugs are necessarily expensive.
Other elements of TG’s model – the avoidance of R&D partnerships and an unprecedented emphasis on the safety of its assets – solve other challenges that small, young companies face. For instance, that combinations of drugs are not only more expensive, but also necessarily more toxic. Or the market wisdom that says that tie-ups with big pharma are validating, value-adding events.
TG Therapeutics was spun out from France-based LFB Biotechnologies SAS in 2010. In April 2011 it took an option from LFB to license rights to ublituximab, a next-generation, glycoengineered, chimeric CD20 antibody. In late 2011, TG reverse merged with Manhattan Pharmaceuticals, and soon after raised $25 million in a private placement and exercised its option to ublituximab (TG-1101).[See Deal][See Deal]
CEO Michael Weiss, former CEO of Keryx Pharmaceuticals, lost no time in licensing in other assets to combine with ublituximab. In August 2012, TG paid an undisclosed up-front to Switzerland-based Rhizen Pharmaceuticals SA to develop its preclinical PI3K delta inhibitor to Phase II, after which it exercised an option for full global rights to the compound, now known as TG-1202.[See Deal] It struck again in June 2014, issuing 125,000 of its common shares as an up-front for a global license to an IRAK4 inhibitor program from Ligand Pharmaceuticals Inc.[See Deal] And in March 2015, it licensed in a PD-L1 and a glucocorticoid-induced tumor necrosis factor receptor (GITR) antibody from Checkpoint Therapeutics Inc., a subsidiary company of Fortress Biotech Inc., for $500,000 up front. [See Deal] The checkpoint inhibitors originated in the lab of Wayne Marasco, MD, PhD, of the Dana-Farber Cancer Institute.
TG’s assets are distinguished by their efficacy and safety profiles and by their complementary mechanisms that allow them to be used in various combinations. The combination of 1101 and 1202, which TG refers to as 1303, is the backbone for further combinations in hematologic cancers and, soon, in autoimmune diseases.
1303 is currently in the Phase III UNITY-CLL trial, under SPA agreement with FDA, where patients are randomized into four arms (1101, 1202, 1303 and standard-of-care) to demonstrate the contribution of each agent to the 1303 combination and to demonstrate progression-free survival (PFS) over Roche's Gazyva (obinutuzumab) and chlorambucil, a standard treatment for advanced chronic lymphocytic leukemia (CLL).
The 1303 backbone figures in another trial, this one at the University of Pennsylvania’s Abramson Cancer Center, the Phase I/II triplet of 1101, 1202 and Merck & Co. Inc.'s PD-1 inhibitor Keytruda (pembrolizumab) in patients with relapsed or refractory CLL. Moreover, 1303 will likely serve as a backbone in TG’s first foray into autoimmune disease; the company plans to start a Phase I/II trial in multiple sclerosis in 2016.
TG’s lead program is the Phase III GENUINE trial of 1101 plus ibrutinib in patients with previously treated, high-risk, chronic CLL. The trial is targeting the end of 2016 to analyze the overall response endpoint; if positive, the company will file for accelerated approval under SPA.
The Era Of The Combination
Combination therapy has been a mainstay in HIV and HCV for several decades. Although it has played a role in cancer, interest in the strategy has been reawakened by deepening knowledge of drug resistance mechanisms, tumor immune surveillance, and how the tumor microenvironment determines the way that tumor cells behave and respond to cytotoxic or targeted agents. Where before, cancer drug combinations were largely a matter of trial and error, they can now be pursued on a more rational basis.
But a greater stimulant to combination therapy has been the recent emergence of checkpoint inhibitors and other immunotherapies. Pharma has primarily pursued partnerships to access assets – immuno-oncology or targeted agents – to test with its internal immuno-oncology candidates. Some of the more notable have been Celgene Corp.’s partnership with Juno Therapeutics Inc. and Pfizer Inc.’s with Merck KGAA. (See .)
The preferred route to oncology combinations has been via partnerships or short-lived clinical collaborations, less so through acquisitions. (See (Also see "In Buzz Of 2015 Pharma Dealmaking, Immuno-Oncology Is Queen Bee" - In Vivo, 16 Sep, 2015.).) Though few dispute the benefits of combining cancer drugs, institutions as varied as the American Society of Clinical Oncology (ASCO) and the Memorial Sloan-Kettering Cancer Center have highlighted the potential of combination therapy to accelerate the unsustainable cost of cancer treatments. Others have decried the severe toxicities that can result from combining targeted therapies or immunotherapies, the 2013 study of the combination of Bristol-Myers Squibb Co.'s Yervoy and Roche's Zelboraf (ipilumumab and vemurafinib) being a case in point. (See (Also see "Yervoy/Zelboraf Combo Trial Fails, But Sequential Study Continues" - Pink Sheet, 4 Apr, 2013.).) And sometimes combinations, for reasons of biology or trial design issues, fail to deliver the expected additive or synergistic benefits. (See (Also see "Cancer Trials & Tribulations: Combinations Are Easier Said Than Done" - Pink Sheet, 1 Aug, 2014.).)
TG thinks it has a better idea. Rather than partner one’s way to what CEO Michael Weiss calls “magical” combinations, TG would focus on licensing in superior oncology assets with complementary mechanisms at relatively low cost.
Rather than partner one’s way to what CEO Michael Weiss calls “magical” combinations, TG would focus on licensing in superior oncology assets with complementary mechanisms at relatively low cost.
The vision required the ability to identify and evaluate the desired drugs. Ublituximab was easy. CD20 antibodies had been in humans for several decades, and their role in depleting B cells was well established. “We had close to perfect information,” says Weiss. The leap here lay in understanding that 1101 was a glycoengineered antibody (meaning its sugar molecules had been manipulated to improve the antibody’s ability to bind to immune effector cells) engineered with low fucose content, which binds to a unique epitope on the CD20 antigen. That made it more potent with respect to antibody-dependent cell-mediated cytotoxicity (ADCC). And understanding what the superior potency could mean for drug combinations, and for patients.
The 1303 backbone consists of superior versions of biologically validated agents, each with a history of use in humans. Ben Bonifant, partner at consultancy Triangle Insights, points out that TG’s strategy of starting with established mechanisms, and building on that with incrementally riskier assets, avoids the potential pitfall of going before FDA with two novel mechanisms and layering the risk.
Gilead Sciences Inc., through its 2011 acquisition of Calistoga Pharmaceuticals Inc., had shown that hitting PI3K delta was a mechanism for regressing tumors. [See Deal]TG put 1202 through rigorous testing at Duke University in serum derived from CLL patients, and verified that it was equal in potency to Gilead’s Zydelig (idelalisib). The preclinical testing also suggested that it would be less toxic than Zydelig, with a longer half-life that would allow QD dosing, a competitive advantage in the class.
When searching for a PI3K delta inhibitor, Weiss says that he passed over some candidates that were more potent than the one he chose. “My rule is that you need to have a threshold of low nanomolar potency but the lowest is not necessarily the best.” Potency is one part of the equation. Extensive toxicity data for 1202 persuaded him to license the drug.
The more recent licenses, to the IRAK4 inhibitor and to the checkpoints, introduced some target risk into the portfolio. These assets were also licensed at an earlier stage, the IRAK4 before toxicity data were available. But research published by Nimbus Therapeutics suggests that IRAK4 is a therapeutic target for diseases driven by aberrant oncogenic MYD88 signaling, and plays a role in B-cell lymphoma and in autoimmune diseases – TG’s sweet spot. [See Deal]Weiss sees a place for it as an add-on perhaps to 1303 in the autoimmune space.
The PD-L1 and GITR assets are antibodies. PD-L1 is a validated target. Although none have been approved yet, several have advanced to late-stage trials. (See ( (Also see "Roche’s Atezolizumab Filing In Sight, With Pivotal Lung Cancer Data In Hand" - Pink Sheet, 17 Aug, 2015.).) TG is confident it has an antibody that engages the target, and doesn’t expect surprises in off-target toxicities. GITR, on the other hand, has not been biologically validated as a target. (See Exhibit 1.)
Exhibit 1
Summary Of TG Therapeutics Portfolio By Level Of Validation
|
Asset |
Licenser, Date |
Stage at License |
Target Risk |
Toxicity Data |
Therapeutic Area Application |
|
CD20 antibody (TG-1101) |
LFB, March 2012 |
PI/II |
Low |
Yes |
B-cell cancers, autoimmune |
|
PI3K delta (TGR-1202) |
Rhizen, Aug. 2012 (Sept. 2014 exercise option for global rights) |
Preclinical |
Low |
Yes |
B-cell cancers, autoimmune |
|
IRAK4 |
Ligand, June 2014 |
Preclinical |
High |
No |
Autoimmune |
|
PD-L1 GITR |
Checkpoint Therapeutics, March 2015 |
Preclinical |
Moderate High |
No No |
Blood cancers |
Strategic Transactions; TG Therapeutics; Word Control
Weiss takes a common sense approach to assembling drug combinations. He feels that some mechanisms – like CD20 and PI3K delta – present obvious combo potential and don’t require overthinking. “You don’t need an advanced degree in molecular biology to think maybe we should try 1101 or 1202 with ibrutinib or with a PD-1.”
TG is a small company of about 35 employees. It relies on vendors and academia for research and manufacturing support, not so much on CROs as it considers clinical trial design and execution a core capability. Sometimes combination possibilities that aren’t so obvious come to it through its research partners. Research it commissioned at Columbia University Medical Center yielded an oral presentation at ASH 2015, the annual meeting of the American Hematology Society, describing impressive synergies in the combination of PI3K delta inhibitor TGR-1202 and Amgen Inc.’s proteasome inhibitor carfilzomib in treating aggressive lymphomas and, potentially, solid tumors. Gilead’s Zydelig and Takeda Pharmaceutical Co. Ltd.’s Velcade, which were also in the mix of possible combinations, did not demonstrate the same level of synergy.
And though TG downplays its science credentials, it relies on a few internal experts and others in its network, as well as its internal database of past combinations, to guide it in rational combos and sequencing, particularly with immuno-oncology assets.
For example: TG had reported preliminary Phase I/II results from its ongoing dose escalation study of 1101 plus 1202 (TG-1303) in heavily pre-treated CLL patients. “There were almost no CLL/SLL [small lymphocytic lymphoma] patients that were not stable or better through two months,” says Weiss. The combo was safe with neutropenia being the only grade 3/4 adverse event greater than 5%.
His team concluded that there is no reason to start the PD-1 inhibitor (in the Phase I/II trial at University of Pennsylvania) at day one. Patients are getting high doses of CD20; they’re just getting up to high doses of PI3K delta; the PD-1 is not needed until the third month. “We think we’ve reduced the tumor burden in all patients by 50% to 70% by the time we start them on an agent that will engage T cells, with the potential for cytokine storm and so forth,” says Weiss. The idea is to introduce the PD-1 into a lower tumor burden environment and give it the best chance of working with the least amount of toxicity.
TG is confident that 1101 and 1202 will remain the backbone for further combinations in both cancer and autoimmune disease. IRAK4 could be an add-on in cancer, but Weiss thinks it will find its utility combined with 1303 in certain autoimmune diseases where it makes sense to add inhibition of the IL-1 pathway to the basic mechanism of B-cell depletion. Same with the checkpoint assets: once they’ve cleared early-stage testing, and the anti-GITR antibody has been validated, the likely plan will be to layer them onto 1303 in hematologic cancers. That’s another potentially “magical” combination.
Avoiding Foreign Entanglements
The second element in TG’s business model, the avoidance of R&D partnerships, while not unique, sets up an unprecedented dynamic in tandem with TG’s proprietary combination strategy.
Weiss takes exception to the view of many investors and sell-side analysts who see marquee partnerships with big pharma as a validating event for a young biotech. He thinks that many young companies, especially those backed by VCs, do deals too early and in some cases destroy future value for shareholders. He concedes that avoiding partnerships could be negatively affecting the way the market values TG Therapeutics. But he also maintains that the smartest investors recognize that deals can be dilutive and not confirming of value.
Weiss thinks the biotech industry is in a cycle where there is a naïve and transient investor base not always up to making its own assessments. In fact, he believes that tapping Wall Street is invariably less dilutive than doing a big partnership early on. He feels fortunate in having strong investors behind him who can fund the company at reasonable valuations. TG currently has about $115 million in cash and equivalents and little debt. It prefers to raise capital through periodic small financings.
But Weiss’ aversion to R&D partnerships and premature commercial deals goes beyond the financial dimension. He believes they are a headache to negotiate and manage, and can often, especially when partnering over a combination regimen, lead to sub-optimal trial protocols. At a recent earnings call, talking about TG’s resistance to partnerships in the cancer business, Weiss said, “We see it as a complicated matrix of what we’re interested in achieving in terms of these combinations, and trying to identify a partner that may share our long-term vision just did not make it interesting for us to explore opportunities on that side.”
Because of the higher cost of building an autoimmune franchise, Weiss will be more welcoming to big pharma partners at an earlier stage.
Cancer is TG’s core business; autoimmune is a complementary revenue stream. TG has the cash and it has the confidence to go it alone with respect to the cancer business. Because of the higher cost of building an autoimmune franchise, Weiss will be more welcoming to big pharma partners at an earlier stage.
In the US, Weiss is comfortable with building out a cancer sales force as TG approaches the market. He’ll make a decision about whether to do so in Europe, or to take a partner; but he’s fine with setting up a commercial organization in Europe if need be. Japan, ROW – he’s open to any kind of partnership that makes sense.
While not keen on partnering, Weiss and his team have been busy on the licensing front, bringing in quality assets for relatively low up-fronts consisting of cash or equity, and modest downstream payments.
It’s The Safety, Stupid!
Although 1101 and 1202 have proven to be as potent or more so than drugs of the same class, TG is counting on their safety profile to differentiate them in the CLL and non-Hodgkin's lymphoma (NHL) landscape. Not only will they be easier on cancer patients, the company hopes that they’ll allow patients – particularly older, frailer ones – to continue longer on treatment. And they’ll be more combinable with other drugs.
Weiss is confident that superior safety will influence payers, particularly when toxicity ends up costing them in hospitalization and expensive interventions. He recognizes that in the most lethal cancers, particularly when efficacy is especially strong, or in second- and third-line settings, that safety can sometimes take a back seat. But he’s counting on oncologists and patients to demand TG’s regimens.
He points out that a lot of PhDs have recently come into the industry. “The industry was more diverse 10 years ago,” he says, “with more MDs and people with good scientific backgrounds but also good common sense.” Scientists tend to be focused on efficacy. They deal with rats and Petrie dishes, and are typically not used to interacting with patients. Weiss speculates that this might make some scientist-executives and even investors less sensitive to risk/benefit considerations that physicians and patients care about.
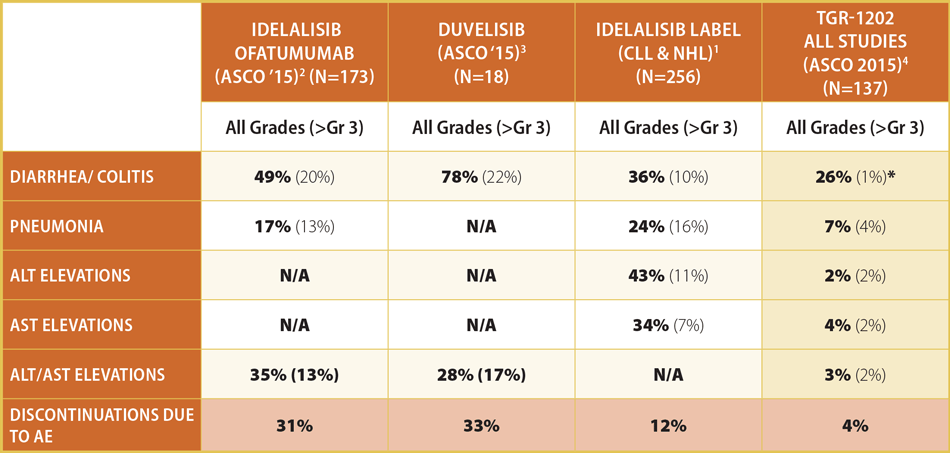
The safety profile of 1202, TG’s PI3K delta inhibitor, especially with respect to discontinuations from hepatic toxicity and colitis, is particularly striking compared with its peers, AbbVie Inc.’s duvelisib and Gilead’s idelalisib. (See Exhibit 2.)
Exhibit 2 Comparative Adverse Event Profile For P13K Inhibitors
(N=66)
* No instances of colitis observed.
Notes: 1) Aggregated from Idelalisib product label; 2) Jones et al, ASCO 2015; 3) Patel et al, ASCO 2015; 4) Aggregated from Burris et al, Lunning at al, Fowler et al, ASCO 2015
SOURCES: TG ASCO 2015 Analyst and Investor Event
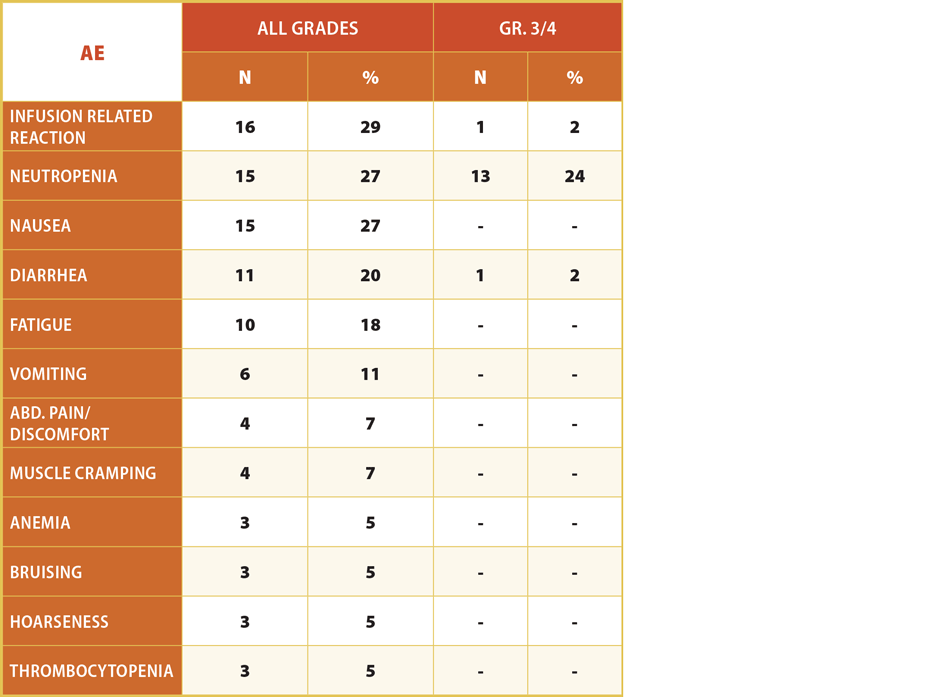
In January 2013, TG initiated a single-agent dose escalation trial of 1202 in patients with relapsed/refractory hematologic tumors. Preliminary data from this study were presented at the June 2015 ASCO meeting. Grade 3/4 adverse events in 66 patients were highest for neutropenia (11%) and anemia (8%); all other AEs were 0% or single digits less than or equal to 5%.
Exhibit 3 Adverse Events Observed In Single-Agent TGR-1202 Dose Escalation Study
(N=66)
Notes: Limited Gr. 3/4 events and no significant dose or time dependent trends in AEs observed with 31 patients on study 6+ months; 3 patients (< 5%) have discontinued due to an adverse event, none of which for hepatic toxicity, colitis, or pneumonitis
TG ASCO 2015 Abstract #7069
TG updated these data in a poster at ASH 2015: in 75 subjects with a variety of relapsed/refractory B-cell malignancies, no grade 3 or greater adverse events were seen in more than 10% of patients. “Incidence of hepatic toxicity and colitis appear significantly less than that reported with other agents in this class,” said Owen O’Conner MD, PhD, of Columbia University Medical Center.
Kicking The Tires On TG’s Model
The test of a new business model is (1) whether it presents a new way to generate returns, and (2) whether it attracts imitators. It’s too early to answer the first question, and the answer to the second is a decisive Yes.
It should be noted that there have been biotech companies in the past that have incorporated at least one of the elements of TG’s model, albeit with significant differences. Ben Bonifant cites Celator Pharmaceuticals Inc., a 13-year-old biotech based on developing synergistic ratios of chemotherapeutic drugs delivered in a proprietary nanoscale vehicle. The company’s lead product, a combination regimen for acute myeloid leukemia (AML), is in a pivotal trial.
TG isn't interested in pursuing a wide variety of tumors, and it’s been cautious in its use of cytotoxic drugs in its combinations. It is more focused on matching complementary mechanisms. Concentrating on a specific class of cancers has allowed it to develop a backbone regimen that works across B-cell malignancies (and potentially autoimmune diseases) yet is benign enough to permit further add-ons. Also, unlike TG, Celator is open to partnerships, research collaborations and out-licensing deals.
Gilead is renowned for developing best-in-class proprietary combinations for HCV and HIV. Moreover, the company’s combination regimens work across the subtypes in each indication, especially HCV, and in that way are similar to TG’s combinations. However, Gilead has not internalized combination therapy to the extent that TG has; it does not appear to be, for instance, a committed feature of its inflammatory or cardiovascular franchises. Also, Gilead has shown itself quite open to R&D alliances, particularly discovery collaborations.
TG was founded on a vision of wholly owned combinations – doublet, triplet and quad – for serious cancers. It found that the best way to pursue that vision was on its own without a pharma partner. And it has taken a novel approach to how it calibrates the balance in its drugs between efficacy and safety. No single element in its model is unprecedented. But working together, particularly in the age of combination treatments, they enable and reinforce one another in a unique way.
The model is certainly vulnerable to imitators. At a Merrill Lynch conference in September 2015, Incyte Corp. emphasized its growing stable of proprietary assets – PD-1, PD-L1, IDO1, FGFR and BRD inhibitors, PI3K delta and JAK1, and preclinical candidates GITR, OX40, TIM-3 and LAG-3. Chief Scientific Officer Reid Huber, PhD, spoke of the company’s intention to test novel combinations of its drugs. Of course, Incyte’s therapeutic focus will be considerably broader than TG’s, ranging over the hematologic and solid tumor landscape.
But TG is also exposed to direct competition to its specific drug combinations and the indications they target. The combo of Gilead’s Zydelig plus rituximab has been approved in the US for relapsed CLL patients; it has shown the following adverse events greater than or equal to grade 3: neutropenia (37%), increased lymphocyte count (18%) and lymphopenia (9%). Serious adverse events occurred in 49% of patients: pneumonia (17%), pyrexia (9%) and sepsis (8%).
Weiss sees the triplets of Zydelig plus bendamustine plus Rituxan and of Imbruvica plus bendamustine plus Rituxan in advanced CLL – both have reported Phase III results – as the primary competition for Imbruvica plus TG-1101 in relapsed/refractory patients, especially in the community setting. He adds, “TG-1303 will compete in both frontline and relapsed/refractory settings.”
Zydelig alone is quite toxic and carries a black box warning for “fatal and serious toxicities” including hepatic, colitis, pneumonitis and intestinal perforation.
The safety profile of TG-1303, from a June 2015 presentation of data from its ongoing Phase I/II trial in 55 heavily pretreated patients appears in Exhibit 3.
Exhibit 4
Safety Profile Of TG-1303 In CLL And iNHL Patients
Related AE’s Occurring in ≥ 5% of Patients (n = 55)
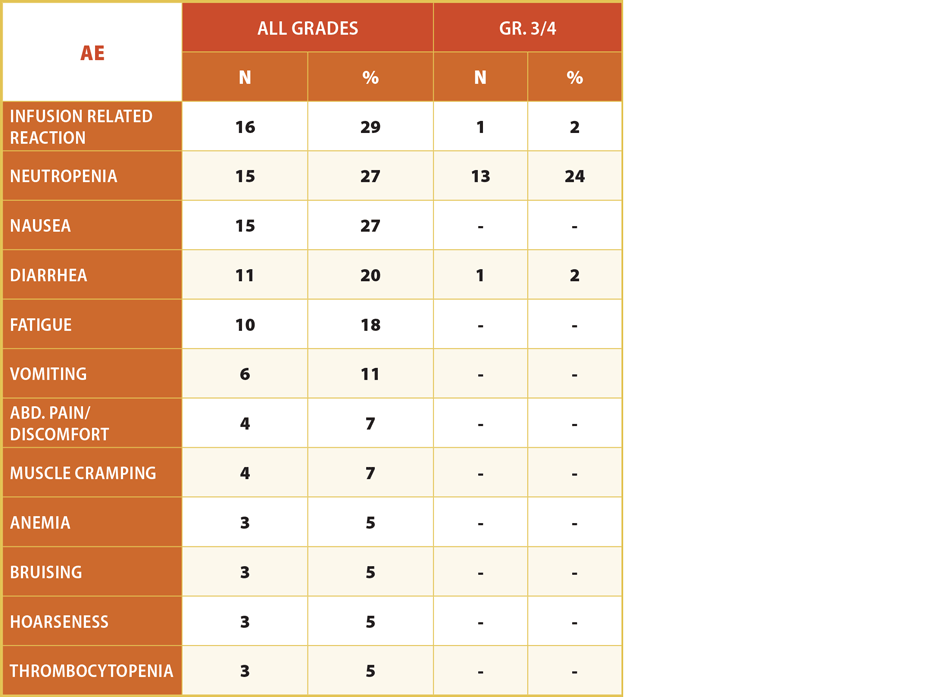
Note: 3 patients (~5%) have come off study due to an adverse event, none related to hepatic toxicity or colitis.
TGTX presentation at ICML conference, June 2015
Bonifant says that TG-1303, should it reach the market, is open to fast followers with an already approved component; all they need do is quickly demonstrate the efficacy of their product with another approved product. Or with one of TG’s components. And oncologists have the option of putting together a similar combination with components they select.
TG’s protection – IP covering its novel combinations is not available to it – is the superior safety of its combinations. That, and the significant head start it has with two Phase III trials running and a registration trial of 1303 in NHL patients on deck, allowing it the time to test add-on improvements to 1303. Its checkpoints, for instance, will likely enter the clinic in 2016, making TG the first company to bring a proprietary triplet including 1303 plus a PD-L1 into the clinic. In fact, the triplet Phase I/II trial of 1303 plus pembrolizumab at the University of Pennsylvania will provide an early read on combining 1303 with a checkpoint inhibitor. So as long as TG stays adept, continues to keep ahead of the pack by initiating new trials and by licensing in complementary assets that are safe and potent, the model stands a good chance of holding up.
For now, Weiss is not emphasizing solid tumors; in fact, the deal with Checkpoint Therapeutics stipulates that TG can apply the assets to hematologic cancers, while Checkpoint Therapeutics reserves their use in solid tumors. However, the company is already exploring the utility of PI3K in solid tumors in a Phase I trial of 1202 as single agent or in combination with nab-paclitaxel/gemcitabine or with FOLFOX.
TG’s next act – building an autoimmune disease franchise – is already in motion. As management disclosed in its third-quarter 2015 earnings call, it will follow the lead of Roche, which recently showed impressive results in late-stage trials of its CD20 antibody ocrelizumab in both primary progressive and relapsing forms of multiple sclerosis. TG will do a Phase I/II trial in 2016 and will likely initiate a Phase III trial in H1 2017. One sequencing option it’s considering is starting patients on IV CD20 and continuing them on oral PI3K therapy, maybe adding an oral IRAK4 inhibitor to the mix.
As for the long game, Weiss is open to the company being acquired. He’s not interested in a dismantling acquisition, where a suitor is drawn just by the PI3K drug or the CD20 in autoimmune disease, and carves those out and shuts down the company. But he would consider an attractive offer by an acquirer who “believed in what we’re doing” and could accelerate it and create a faster, bigger platform. “We would work within that structure, and hopefully be able to continue to do what we’re trying to accomplish.”