France Seeks Stronger Cost-Effectiveness Tools To Lower Drug Prices, But Pan-European Bulk Buying May Come First
France’s drugs assessment agency wants simpler, stronger rules to evaluate the cost-effectiveness of new drugs. But changes to the country’s complex reimbursement system may be too slow to address immediate budget concerns, making pan-European bulk buying appear a more likely near-term solution.
In the light of the “colossal budgetary impact” of new medicines, France’s drug assessment and reimbursement agency, the Haute Autorité de Santé, is seeking to simplify, strengthen and clarify its evaluation methods, including those used to determine cost-effectiveness. The goals: a tighter link between cost-effectiveness and price, and more robust tools to measure the cost of innovation – without resorting to the kind of UK-style rationing that can lead to certain new therapies being denied reimbursement.
Indeed, although the latest version of France’s Social Security and Finance Bill, currently making its way through parliament, mandates shaving €1.6 billion ($1.7 billion) off the drugs bill, France still isn’t ready to adopt a cost per quality-adjusted life-year (QALY) threshold like the National Institute of Health and Care Excellence (NICE) in the UK. “That’s not realistic for France on the short term,” says Catherine Rumeau-Pichon, assistant director at HAS. She cites the need to take into account other factors such as unmet need and population size. Unsaid is the fact that a threshold remains politically unacceptable in this socialist country. And HAS, though nominally independent, has a government-appointed board and can only propose system changes; it can’t implement them without legislative backing.
What’s more, health technology assessment of any kind is relatively new in France: the country has only been carrying out cost-effectiveness analyses of individual drugs and devices since 2013. Before that, HAS looked almost exclusively at their clinical and medical benefit. The agency still doesn’t touch drug pricing – that is negotiated with drug companies by a separate body, the Comité Economique des Produits de Santé (CEPS). But, especially since the economic shock waves incurred by the arrival of Gilead Sciences Inc.’s revolutionary hepatitis C treatment Sovaldi (sofosbuvir) in 2014, HAS is talking more freely about cost, and about QALYs, that standard currency of health benefit. Indeed, the enormously wide variation in cost per QALYs calculated by HAS’ health economics division to date was cited by HAS as a key reason for its solicited changes. “The [costs per QALY] go from less than €10,000 to more than €200,000,” states an October 2015 release calling for the re-think.
If a threshold isn’t on the books, “more explicit advice” from HAS to the pricing authority, which negotiates with drug manufacturers, is, according to Rumeau-Pichon. She wants price negotiations aimed specifically at lowering a product’s incremental cost-effectiveness ratio.
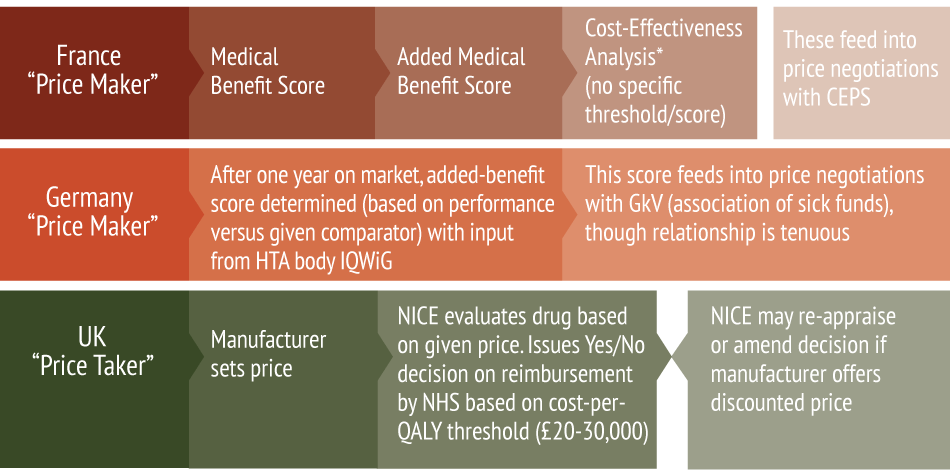
In other words, HAS wants to sharpen the claws of its health economics arm (known as the Commission Evaluation Economique et de Santé Publique, CEESP). But it wants to do so without moving away from France’s negotiation-focused approach to drug pricing. Rumeau-Pichon refers to this as a “price-maker” approach: France’s system feeds clinical and cost analyses into subsequent price negotiations with pharma, whereas the UK, in contrast, takes a manufacturer’s requested price to calculate a cost per QALY, thus establishing what will or won’t be reimbursed. This “price-taker” approach can, and often does, lead to the UK’s refusal to reimburse certain products. (See (Also see "Calls For NICE Reforms Intensify After Early Enzalutamide Use Rejected" - Pink Sheet, 12 Jun, 2015.).) (See Exhibit 1.)
Exhibit 1 Price-Makers And Price-Takers: France, Germany And UK Compared
*for specific products.
IN VIVO research
So how does HAS propose to improve “price-making,” without brandishing the big stick of reimbursement refusal (at least for innovative drugs; me-toos or those deemed of insufficient medical benefit may already be denied reimbursement)?
It’s not yet clear. But some answers may lie within an extensive report by economist Dominque Polton, a director at the French national insurance fund for salaried workers (CNAMTS). This document, commissioned by health minister Marisol Touraine early in 2015 and officially received in mid-December, critically assesses the criteria and methods currently used to value medicines, including the role of the CEESP. It has much to say about the system’s shortcomings, including a lack of coordination and understanding between the various decision-making bodies. It duly proposes a more prominent role for cost-effectiveness analyses; clear, published reports using accessible language; and tighter integration of the CEESP with the clinical/medical evaluation commission. It also calls for more systematic measurement of the budgetary impact of new medicines.
The Polton report also proposes some more fundamental shifts to the structure of France’s underlying clinical evaluation process and reimbursement rules, however. And that’s no wonder, says a source from within HAS, because the whole lot is inextricably linked. “If you start to change the assessment criteria, you are forced to look at reimbursement rates,” explains the source. France’s current drug evaluation system involves two scales: one to determine absolute medical benefit (whether a drug or procedure will be reimbursed or not) and another additional medical benefit (which determines the rate of reimbursement: 15%, 30% or 65%). (See sidebar, "France’s Drug Reimbursement System.") Neither addresses price.
HAS has for years been seeking to merge these scales into a single, simpler index that strongly emphasizes comparative effectiveness (the index thérapeutique relatif, ITR). (See (Also see "Europe’s Future HTA Landscape: More Converged And Cost Conscious" - Pink Sheet, 14 Jan, 2013.).) Acknowledging this (and the fact that the ITR was considered overly rigid and formulaic), Polton proposes a relative therapeutic value score to replace the current additional medical benefit measure, with fewer, more clearly defined scoring categories. It also puts forward a single reimbursement rate (suggested at 60%) and eventually an end to all reimbursement for products showing limited medical benefit. Such a set-up would be “more coherent, simpler and more in line with other countries’ practices,” Polton was reported as saying.
Health minister Touraine is expected to announce “within weeks” any measures that may be implemented as a result of this report. Moving to a single reimbursement rate doesn’t look likely, though, not least due to the public outcry that may result from de-reimbursing certain older products. “We’re not going to fundamentally change the system,” insists Rumeau-Pichon. She does foresee more performance-based pricing deals, though, and (as signaled in the report) more rigorous demands on drug firms, including sensitivity analyses using a wider range of pre-specified prices. This is still subject to discussion, however. And the report doesn’t deal directly with what is arguably the heart of the matter: how the pricing authority CEPS uses the clinical- and cost-effectiveness analyses supplied to it in negotiations with industry.
In short, nothing is likely to happen fast. Not least since HAS is facing its own existential crisis: its president (and head of the health economics committee) Jean-Luc Harousseau in September 2015 unexpectedly announced his resignation, more than a year before his term was due to end. He cited personal reasons. Sources suggest a poor relationship with the health minister may have also contributed: Touraine was allegedly dissatisfied with the analyses and material HAS provides to CEPS for pricing discussions – which would explain why she commissioned the report. Whatever Harousseau’s true reasons for quitting, questions remain about HAS’ authority, resources and methodological expertise in the face of continued, pressing questions around public health – not least how to pay for it.
More Inter-National Drug Purchasing In Europe
In the meantime, inter-national drug purchasing may prove the more effective and immediate, if rather blunter, method for lowering prices in some continental European nations. When Sovaldi hit the European markets, it was health minister Touraine who sought out inter-national collaboration to get the best price. That teamwork didn’t materialize for that drug – the proposal came too late to allow implementation, according to a source close to the discussions. “But that the suggestion was even made shows that things are moving,” the source continues.
Belgium and the Netherlands have already agreed to pilot joint-pricing talks for rare diseases drugs starting in 2016, boosting each small nation's negotiating position. (See (Also see "European Notebook: Drug Pricing Collaborations; Biosimilar Switching; Scientific Advice" - Pink Sheet, 27 Apr, 2015.).) “The wind is blowing in the direction” of more European-level price negotiation, concurs Rumeau-Pichon, though she’s quick to add that national-level reimbursement and pricing systems complicate matters. In an impactful October 2015 Forbes![]() article examining drug pricing, Jack Scannell, an associate at the Innogen Institute and associate fellow at the Oxford, UK-based Centre for the Advancement of Sustainable Medical Innovation (CASMI), writes, similarly, that “European countries should buy as a block … and run competitive tenders to find the cheapest supplier for pan-European demand.”
article examining drug pricing, Jack Scannell, an associate at the Innogen Institute and associate fellow at the Oxford, UK-based Centre for the Advancement of Sustainable Medical Innovation (CASMI), writes, similarly, that “European countries should buy as a block … and run competitive tenders to find the cheapest supplier for pan-European demand.”
Scannell stamped his suggestion with a label of "hopeful implausibility." But as more targeted, innovative therapies reach the market, and absent the political will to say "no," bulk buying may prove the least complex solution. Indeed, “organizing buying power is the only way to come to any kind of negotiating position,” declares Ad Schuurman, head of international affairs at the Netherlands’ health technology assessment and reimbursement agency. “Without it, it will be ‘take it or leave it’ for patients and health care systems,” he says. A further tailwind for bulk buying: the Netherlands will hold the EU presidency during the first six months of 2016.
France negotiated one of Europe’s lowest prices for Sovaldi in 2014, without the help of any neighbors. But it did so by invoking a special additional tax on sales of companies selling HCV drugs, ushered into legislation at the last minute. Conjuring up a tax for each new expensive medicine is hardly a long-term solution to funding innovation.
Nor, deplores Les Entreprises du Médicament (LEEM), France’s drug industry association, is hitting drug costs for over half of the €3 billion health care budget cuts outlined in the new finance bill. The association, currently negotiating its next three-year umbrella contract with CEPS, is instead calling for fundamental structural reforms to the country’s health system, and for a broader focus on the downstream cost-savings that proper use of medication can allow. It’s a familiar refrain from the drug industry everywhere. But while drug prices remain in the hot seat, it’s falling on deaf ears.
LEEM wasn’t willing to comment on the suggested drug valuation and reimbursement changes prior to the official release of Polton’s report.