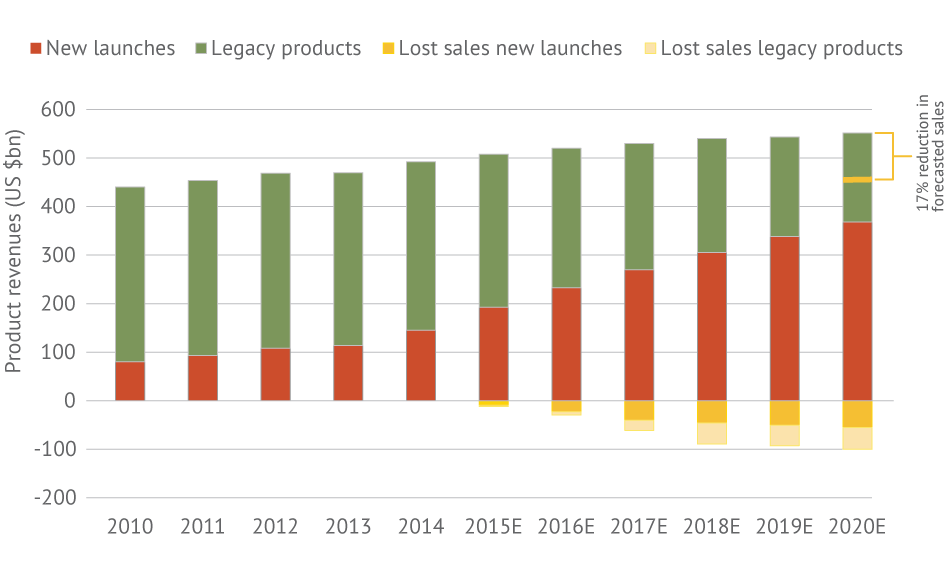A Road Map To Strategic Drug Pricing
Executive Summary
The current unit-based pricing model for drugs is too one-dimensional for the market's present needs. Pharma firms must identify products that will benefit from innovative pricing models, and then forge the types of collaborations that will support those models.
- Current pricing practices create conflict between drug companies and other health care stakeholders, fostering a negative reputation for the biopharmaceutical industry and a slowdown in growth.
- Because products come to market with clinical trial data and not real-world evidence, stakeholders may see them as having “potential,” not "proven," value at the time of launch.
- As a result of this evidentiary divide, many products already enter the market with a “value gap.”
- To accelerate the shift to proven value and bridge the value gap, biopharma companies should consider multi-stakeholder collaborations aimed at co-creating data to support innovative pricing models.
- EY’s qualitative pricing methodology helps companies understand which products will derive the greatest benefit from innovative pricing models, enabling a proactive and systematic approach to pricing decisions.
The debate about drug pricing has reached a fever pitch. In early February 2016, the US Congress held a half-day hearing on pharmaceutical pricing. Long on spectacle and short on solutions, the meeting was a reminder that even in the US, the most “free” market for drug prices and access, there is widespread concern about the impact of rising drug costs on the sustainability of health care spending. Instead of viewing drugs as one of the most efficient and cost-effective solutions to illness, it’s clear the public views biopharmaceuticals – and the companies that make them – as one of the central problems contributing to an affordability crisis.
It is time to acknowledge that our historical pricing model, which is built on unit-based pricing, is too one-dimensional for the marketplace’s current needs. It has resulted in incentives that encourage biopharma companies to make pricing decisions that are driven by what is possible rather than what other stakeholders consider reasonable. It should be no surprise, then, that when important therapies for life-threatening diseases reach the market, these products frequently come with budget-straining price tags. In the US, the current pricing dynamics have also enabled annual (or in some cases, biannual) price increases for products already on the market.
Admitting “we’ve done things we shouldn’t do,” Leonard Schleifer, CEO of Regeneron Pharmaceuticals Inc., told the audience at the 2015 Forbes Healthcare Summit in December the industry has “to think about a different pricing approach that is a little bit more responsible.”
In truth, there won’t be just one pricing approach, but many. The strategies that will be implemented will depend on the competitive intensity of the therapeutic area, the economics of the individual market and specific product attributes. Moreover, given the complexity and time required to implement new pricing models, not every drug in a portfolio will be worth such investment. When, and how, should biopharma companies place their bets?
We outline a qualitative methodology designed to help biopharma leadership teams proactively identify when to adopt novel pricing strategies. The truth is many companies take an overly transactional view of market access, viewing stakeholder engagement as a negotiation game. In this context, innovative, value-based pricing collaborations are more commonly seen as a defensive hedge, deployed only when reimbursement is delayed. However, as pricing pressures grow and the evidentiary demands increase, more products, not fewer, will require innovative pricing strategies.
Instead of defaulting to unit-based pricing methods, companies need a more systematic approach that helps identify, across a portfolio, which products should be candidates for innovative solutions in the different markets where they will be sold. To work, this approach must be grounded in an honest assessment of how other stakeholders, especially the payers, value the medicine’s different features.
Getting there won’t be easy. There will be new business risks and real implementation challenges. For starters, biopharma companies must identify which stakeholders are most ready to embrace these more collaborative pricing models. In addition, manufacturers must work with stakeholders to define what is meant by an “outcome” and develop the infrastructure to capture and analyze the data.
For biopharma companies to meet their future growth objectives, they must embrace holistic pricing solutions now before payers use blunt methods to curb costs and limit patient access.
But biopharma companies must also acknowledge that maintaining the status quo comes with significant business risks. Because of cost constraints, infinite resources to support access to innovation no longer exist. For biopharma companies to meet their future growth objectives, they must embrace holistic pricing solutions now before payers use blunt methods to curb costs and limit patient access.
A Model Under Increasing Pressure
The economic drivers that guide the pricing of televisions, mobile phones or clothing don’t apply to the pricing of drugs. There are multiple reasons for this, including market exclusivity and a disconnect between the economic buyer (the payer) and the end user (the patient). But the primary reason for high drug prices stems from the structure of the current system, which relies on unit-based pricing, a methodology that needs to evolve as the larger health care ecosystem itself evolves.
Biopharma companies have responded to the existing market incentives in rational and predictable ways. They have established public, unit-based list prices for products and then negotiated, on a market-by-market basis, specific, undisclosed discounts or rebates based on in-country regulations and health technology assessment criteria. This approach has had two benefits: 1) it is relatively simple to implement; and 2) it preserves pricing flexibility, especially in markets where reference pricing is the norm.
In the past, this lack of net pricing transparency worked to manufacturers’ advantage. However, in today’s environment, where the list prices of drugs are high and publicly available, the public doesn’t discriminate between the perceived cost of a medicine and the amount actually spent. Moreover, the heterogeneity of drug costs globally – for instance, certain cancer drugs can cost half as much in Europe as in the US – reinforces perceptions that pricing practices are “unfair,” fueling industry’s negative reputation.
Biopharma’s historical pricing model is now under threat. One reason: the temporal misalignment between when drug costs occur and when the benefits are realized. Companies must be rewarded for the difficult and risky work of developing new drugs. But this means many specialty products come with high up-front price tags. Resource-constrained payers, however, need drug utilization policies that are consistent with tight annual budget cycles. With very few exceptions, the benefits associated with a therapy won’t be measurable until many years in the future. As Kenneth Frazier, the CEO of Merck & Co. Inc., noted at a November 2015 forum sponsored by the US Department of Health and Human Services, “the value of a drug is like an annuity. The issue for the health system is the return on investment needs to be made up front.”
Hit hard by their own budget constraints, payers are therefore adopting new restrictions that limit the use of newly launched products. As multiple drugs with similar indications and clinical impact compete for share in therapeutic battlefields such as oncology or diabetes, it can be difficult to differentiate newer entrants from existing players. A flood of biosimilars creates additional downward price pressure in categories that have historically enjoyed pricing flexibility.
In this environment, steep discounts and aggressive rebating strategies to establish market access have become the norm. The more comparable the drugs, or the greater the number of competitors in a particular market, the greater the likelihood companies find themselves sacrificing pricing power – and future revenues. (See (Also see "Game’s Up, Pharma: The New Drug Pricing Dynamics" - In Vivo, 19 Mar, 2015.).)
We’ve seen it already. Recall what happened in 2014 after AbbVie Inc. launched Viekira Pak (ombitasvir/paritaprevir/ritonavir tablets; dasabuvir tablets), an alternative to Gilead Sciences Inc.’s all-oral hepatitis C regimens Sovaldi (sofosbuvir) and Harvoni (sofosbuvir/ledipasvir). As Gilead noted on its February 2015 earnings call![]() , the presence of a competing product put pressure on the Foster City, CA-based biotech to offer larger discounts to keep its products on payers’ formularies. The near-simultaneous launches of two new PCSK-9 inhibitors in mid-2015 provided another signpost of payer behavior: payers delayed coverage decisions until both products were approved in order to leverage competition in the marketplace when negotiating access to this class of drugs. The upshot: slower-than-anticipated sales for both products.
, the presence of a competing product put pressure on the Foster City, CA-based biotech to offer larger discounts to keep its products on payers’ formularies. The near-simultaneous launches of two new PCSK-9 inhibitors in mid-2015 provided another signpost of payer behavior: payers delayed coverage decisions until both products were approved in order to leverage competition in the marketplace when negotiating access to this class of drugs. The upshot: slower-than-anticipated sales for both products.
Modeling by EY suggests that even as biopharma companies deliver on their R&D pipelines, payer restrictions could eliminate $100 billion in newly launched and existing product revenues by 2020.
Recent analysis by the industry association PhRMA suggests payer pushback has already negatively affected revenue growth across the industry. In its 2015 report “Prescription Medicines: Costs in Context![]() ,” PhRMA estimated that net brand price growth for biopharma products fell from a high in 2012 of $16.8 billion to a low in 2014 of $10.3 billion as a result of increased rebates and price concessions.
,” PhRMA estimated that net brand price growth for biopharma products fell from a high in 2012 of $16.8 billion to a low in 2014 of $10.3 billion as a result of increased rebates and price concessions.
What if the situation worsens in the coming years, as drug costs become a bigger line item in national budgets? Modeling by EY suggests that even as biopharma companies deliver on their R&D pipelines, payer restrictions could eliminate $100 billion in newly launched and existing product revenues by 2020. That’s about 17% of forecasted sales. (See Exhibit 1.)
Exhibit 1 Impact Of Payer Skepticism Payer skepticism of value could reduce 2020 biopharma revenues by $100bn.
Note: From 2015 to 2020, sales of newly launched products are forecast to have an imputed 17% compound annual growth rate (CAGR), while sales of legacy products are projected to decline by an imputed 9% annually. To model the potential payer pushback, EY assumed the CAGR for sales of new launches slowed modestly to 14%; EY also assumed the annual decline in legacy product sales increased modestly to 13%. Together this mix of potentially slower than projected growth from new launches and accelerating erosion from legacy drugs represents about $100 billion in lost product sales, roughly half of which would be felt by big pharma companies.
SOURCES: EY; Decision Resources
Value Is In The Eye Of The Beholder
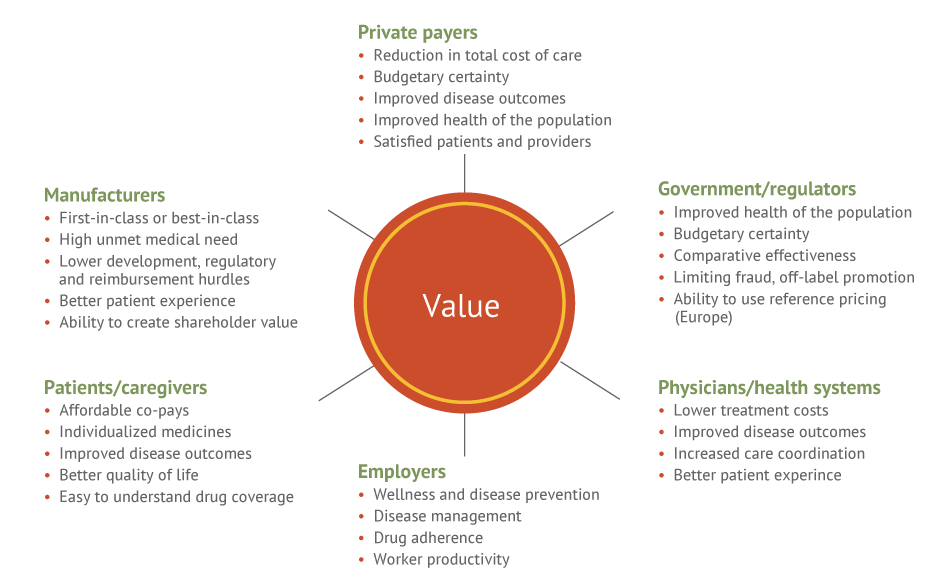
A critical challenge when developing balanced pricing strategies is the fact that there is no single arbiter of product value. The health care marketplace is populated by several different types of stakeholders, each of which defines value and influences prescribing decisions slightly differently. (See Exhibit 2.)
It’s still true that stakeholders value product efficacy and safety, but as with improvements in quality of life, these attributes should be considered necessary but not sufficient. In today’s increasingly fee-for-value world, value drivers embraced by European health systems have emerged as drivers of acceptability in the US:
- Significant differentiation compared with the standard of care
- The ability to subsegment the population most likely to benefit
- Real-world outcomes
- Up-front affordability of the medicine
- Total cost to the health care system
- Time required to achieve cost savings
Even in Europe, where health technology assessment organizations delineate value via clinical effectiveness and cost-effectiveness, there is no standardized value definition. Not only do the value formulas vary from country to country, but how those formulas are implemented within a given market may be inconsistent. In the US, where there is even greater payer fragmentation and it has been politically intolerable to use cost-effectiveness measures to determine drug prices, it is even more difficult to reach a universal viewpoint on the subject.
That doesn’t mean payers stateside are disinterested in objective frameworks to define the concept, however. Thus, in 2015, one of the key new developments in the value discussion was the proliferation of third-party tools that compare the efficacy, side effects and costs of different products. (See (Also see "Scoring Value: New Tools Challenge Pharma's US Pricing Bonanza" - In Vivo, 21 Oct, 2015.).)
Exhibit 2 Value Is In The Eye Of The Beholder
EY
Whether these value frameworks originate from health technology assessment organizations or private groups, their existence directly affects the pricing of biopharmaceutical products. That’s because these different assessments provide credible pricing alternatives that manufacturers must address head on when trying to justify a product’s value.
Absent credible alternative data about product value, payers will use the information gleaned from such tools to demand deeper and deeper discounts in the marketplace. Such payer behavior ultimately limits biopharma value creation, turning drugs into commodities and manufacturers into vendors.
Moving From Potential To Proven Value
Although biopharma companies amass considerable efficacy data during clinical trials to support regulatory decisions, these data don’t necessarily demonstrate real-world value – that requires evidence outside a clinical trial showcasing improved outcomes against the current standard of care.
With multiple therapeutic options available in almost every drug class, a majority of products now coming to market will be classified as having “potential value” until there is proven evidence. As a result, at launch, many products must bridge an evidentiary “value gap.” Because of their high price tags, this gap is especially pronounced for specialty medicines.
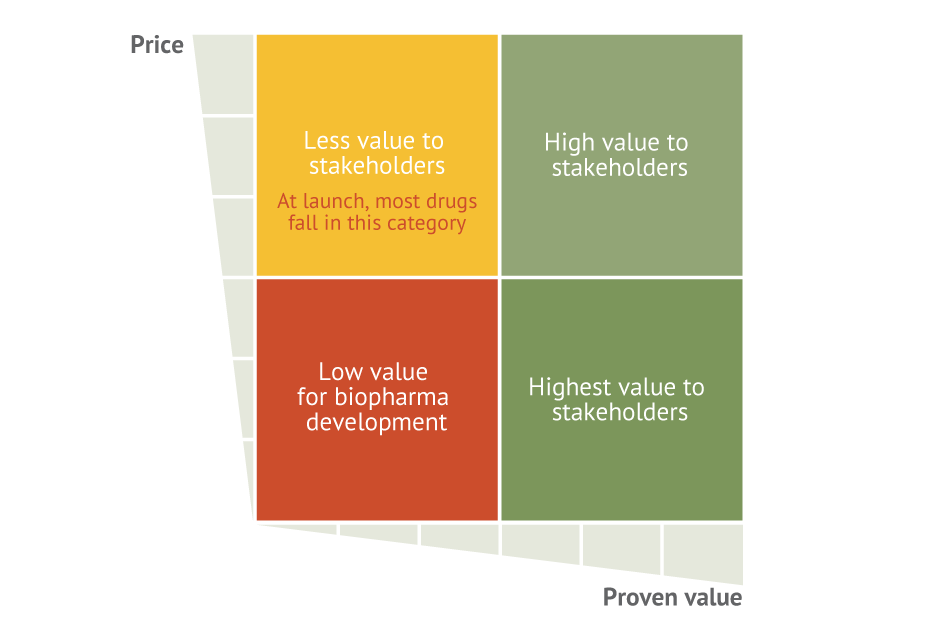
Indeed, as Exhibit 3 illustrates, stakeholders typically categorize newly launched drugs into one of four categories based on existing data:
- High price/high value product
- High price/low value product
- Low price/high value product
- Low price/low value product
Exhibit 3 Categorizing Newly Launched Drugs At launch, drugs map to one of four categories based on stakeholders’ perceptions.
EY
High price/high value products include curative therapies such as the all-oral hepatitis C regimens and medicines that provide a step change in the standard of care. These medicines are of high value to stakeholders but, because of the up-front costs, raise concerns about affordability.
High price/low value medicines include specialty products that are undifferentiated relative to standard of care or me-too products that offer incremental improvements in efficacy or real-world outcomes. This category may also include chronic disease products that treat broad populations but are not well targeted. Thus, although the therapeutic may be very effective in a subsegment of the population, the observed efficacy in the broad population may be underwhelming because a majority of patients are non-responders. Products in this category are most at risk for pushback from payers and skepticism from providers and patients since benefits achieved relative to their costs are harder to determine.
Low price/high value products include vaccines and generics and are viewed by stakeholders as having the greatest utility because the benefit/cost ratio is highest. Even products in this category, however, may be susceptible to up-front affordability concerns, depending on the macroeconomic conditions of the market and the number of patients affected.
Low price/low value therapeutics, which include over-the-counter medicines and topical ointments, traditionally hold the least value because their therapeutic benefits can’t be broadly attributed across the population. For pharmaceutical manufacturers, these products have been viewed as the lowest development priority because the likely returns are lower relative to their development and commercial risks.
Inching Toward Innovative Pricing
Pricing approaches of the future will require companies to work with other stakeholders, especially payers, to co-create data that bridge the value gap. To be most effective and accelerate the shift from potential to proven, these data will ideally be collected not just after launch but during development. Thus, companies serious about innovative pricing strategies must also rethink their organizational structures to establish closer relationships between the product development and commercial strategy teams.
Change is already under way, albeit on an ad hoc basis: payers and manufacturers in different markets are experimenting with a number of innovative pricing models that represent a shift from unit-based pricing. (See Exhibit 4.) In Italy, for example, access to most high-priced oncology products requires some kind of pay-for-performance arrangement that necessitates monitoring via patient registries. In the UK, financially based risk-sharing agreements have become the preferred approach, in part because of the complexities and costs associated with creating effective outcomes-based contracts.
Exhibit 4 Moving To Fee-For-Value: Selected Solutions
|
Solution |
Definition |
Use In Marketplace |
Example |
|
Indication-specific pricing |
Differential product pricing depending on its performance in specific indications (e.g., lung versus head and neck cancer) |
Emerging |
Express Scripts pilots program to test indication-specific pricing |
|
Bundled payment |
A global payment for all treatment costs, including prescription drugs |
Procedures and physician services: high Therapeutics: emerging |
United Healthcare Group partners with multiple physician groups |
|
Financial-based risk sharing (FBA) |
Agreement links price to utilization (either via script volume or drug dosage) Agreement provides budgetary certainty to payers |
Europe: high US: emerging |
Gilead Sciences and government of France agree to a volume-based cap on Sovaldi |
|
Performance-based risk sharing (PBA) |
Agreement helps manage utilization and/or provide evidence of drug efficacy Agreement provides payers with clinical outcomes data |
Europe: medium US: emerging |
Bristol-Myers Squibb and Italian government establish PBA for Yervoy that includes payment-by-result |
|
Annuity model |
Financing instrument covers the acquisition cost of breakthrough biopharmaceutical products Instrument can be structured as bond, mortgage or credit line Pay-for-outcomes agreement likely to be a component |
Emerging |
Health impact bonds used to improve care delivery for chronic diseases such as asthma To date, life sciences companies have not participated in creation of such instruments Could be important future solution for high cost, curative therapies |
SOURCES: EY; Company reports; French Ministry of Social Affairs and Health; Italian Medicines Agency
In the US, there has been more limited experimentation with innovative pricing, due to concerns that novel pricing arrangements would jeopardize government contracts and regulations related to Medicaid price. Still, budgetary pressures stateside mean payers and drug companies have increased motivation to make value-based contracts work.
Indeed, by the end of 2015, biopharmas had struck at least seven novel pricing arrangements with payers, according to publicly sourced documents. Novartis AG is one of the most vocal proponents of new pricing models; the Swiss pharma hopes to use outcomes-based pricing to enable greater access to its first-in-class congestive heart therapy Entresto (sacubitril/valsartan). Thus far, only Aetna Inc. and Cigna Inc. have disclosed novel contracts for Entresto, which Novartis acknowledges has had slower-than-anticipated sales due to reimbursement delays. (See (Also see "Novartis On Payer Contracts, Other Updates From BIO CEO & Investor Conference" - Pink Sheet, 15 Feb, 2016.).)
Based on a combination of market- and product-related attributes that take into account the actual payer in question, our approach identifies which factors are most likely to have the greatest impact on a company’s ability to achieve maximum pricing flexibility ahead of a new product launch.
EY’s Strategic Pricing Methodology
In a general way, the categories described above help segment products based on the views of payers and other stakeholders. To discriminate between products that are better suited for innovative pricing models and those that can be supported by traditional pricing strategies, a more systematic analysis is required. Thus, EY has developed a qualitative, three-step strategic pricing methodology.
Based on a combination of market- and product-related attributes that take into account the actual payer in question, our approach identifies which factors are most likely to have the greatest impact on a company’s ability to achieve maximum pricing flexibility ahead of a new product launch. As a result, a biopharma can preemptively develop specific tactics, including targeted data collection and novel contracting mechanisms, to maximize the value creation – and minimize the uncertainty – associated with any specific attribute. In this way, the model accelerates the shift from potential to proven and closes the value gap.
When applied across the entire portfolio, companies can use the methodology not only to tailor the right pricing approach to the right product, but also to improve strategic business decision-making. Moreover, the methodology is flexible enough to adapt to evolving market conditions, including rapidly changing definitions of the standard of care. (See sidebar, "Applying The Methodology.")
The three steps in the process are:
- Assess the market and product attributes.
- Confirm the pricing analysis.
- Tie the pricing strategy to the commercial strategy.
1. Assess market and product attributes
To accurately determine a product’s pricing flexibility at launch, a company must first assess a number of attributes that are both market- and product-specific. Eight different factors play a role in determining how much pricing flexibility a company will have when launching a particular product. (See Exhibit 5.)
Exhibit 5 Market- And Product-Specific Attributes Determine Pricing Flexibility
|
|
Attribute |
Definition |
Impact On Pricing Flexibility |
|||
|
Market-specific |
Competitive intensity |
Assesses number of therapies on the market to treat the disease |
Pricing flexibility increases the fewer the number of competing products |
|||
|
Economic burden of disease |
Examines the potential budgetary impact of the therapy to the stakeholder |
Pricing flexibility increases the lower the up-front costs associated with treating a disease |
||||
|
Disease severity |
Evaluates the seriousness of the disease |
Pricing flexibility increases with disease severity given the high level of unmet medical need
|
||||
|
Payer archetype |
Considers how different behaviors motivate payers to make drug coverage decisions as well as willingness to engage in novel types of contracting |
Pricing flexibility increases if the payer is focused on wellness and prevention rather than cost and has a stable membership population. Such payers are also more likely to engage in innovative pricing models |
||||
|
Product-specific |
Differentiation |
Measures a product’s effectiveness relative to available treatments, especially standard of care |
Pricing flexibility increases if the product provides a step change in care relative to the competition |
|||
|
Time to outcome |
Analyzes the time required to demonstrate effectiveness to the stakeholder |
Pricing flexibility increases the shorter the time to a credible real-world outcome, including a demonstrable cost-offset |
||||
|
Degree of targeting |
Measures the therapy’s use in population subsegments |
Pricing flexibility increases when precision medicine tools narrow the population from all comers to responders. Such targeting not only improves outcomes but addresses the budgetary concerns of payer stakeholders |
||||
|
Patient experience (e.g., a dosing schedule that facilitates adherence to therapy) |
Assesses a therapy’s impact on quality-of-life metrics and potential costs of switching to alternate therapies |
Attributes may be of greater importance to patients than traditional payers. Thus, patient-centric attributes are unlikely on their own to result in pricing flexibility; real-world data demonstrating differentiation relative to the standard of care will be important |
||||
EY
Given the current complexity of drug pricing and the diversity of payer types, it is difficult to rank order the eight factors in a decision tree that holds true across all therapeutic areas. Instead, depending on the severity of the disease, the total projected costs of treating the indication and the competitive intensity of the market, certain attributes will be more central than others in determining a product’s pricing flexibility.
As a result, this assessment provides directional guidance about not just how to price a product, but also where the biggest evidence gaps reside. Notice that a high degree of uncertainty around any one attribute increases a stakeholder’s skepticism, and thus, the likelihood that there will be a value gap at launch. By understanding which factor results in the greatest uncertainty, a company can proactively develop data to address the stakeholder’s concerns. In effect, this attribute becomes the fulcrum for stakeholder engagement around new pricing models.
There is little that companies can do to influence the competitive intensity of the therapeutic area or the severity of a given disease. At a strategic level, companies must decide if these attributes make a particular disease attractive for drug development more generally.
If a new product is a late entrant into a class with multiple established products (e.g., high competitive intensity), it will be imperative to differentiate the product in head-to-head trials against the comparator stakeholders determine to be most relevant. This may be a product with a similar mechanism of action; alternatively, it may be a much cheaper generic, or even a device or digital app. In today’s value-oriented world, the most relevant comparator is the one that currently provides not only the best health outcome but is also affordable.
Novel pricing strategies can play a critical role in facilitating uptake in a number of instances, including when the economic burden of the disease is high and the time to outcome is long. Drugs aimed at larger swaths of the population will incur greater up-front costs. The higher these costs to other health care stakeholders, regardless of the demonstrated outcomes, the higher the likelihood that pricing decisions will generate scrutiny. This has been the case for the all-oral hepatitis C regimens that are curative. Similarly, therapies that require a longer time to demonstrate a real-world outcome, including demonstrable cost offsets, will be subjected to more stakeholder skepticism than products that demonstrate outcomes quickly.
Finally, the nature of the buyer, the payer archetype, is another critical issue when considering a novel pricing strategy. Different types of payers are motivated to make different coverage decisions based on their individual preferences and constraints, including the market dynamics in which they operate. In the US, for instance, Medicaid payers are very focused on up-front medication costs because of fixed budgets. Integrated delivery networks, however, might be less sensitive to up-front costs if the medicine results in credible cost offsets in an acceptable period of time. Note, since integrated delivery networks traditionally keep their members for long periods of time, this particular type of payer may have more flexibility on the time-to-outcome parameter than a traditional commercial payer who will have the patient as a member for only one or two years.
Because of these behavioral differences, the payer archetype will likely influence a range of factors, including whether or not a given payer is open to an innovative pricing strategy in the first place. Given the complexity of these collaborations and the required investments in time and capabilities, it makes sense for companies to engage first with payers that are most receptive to novel contracting arrangements.
Once companies have identified payer partners, they will also have to determine which market and product attributes are of greatest importance to that particular organization. Here again, the payer archetype is likely to play a role. Indeed, an analysis of five recent outcomes-based contracts in the cardiovascular space illustrates the diversity of endpoints that can be considered: adherence to therapy, cholesterol lowering and a reduction in cardiac events or hospitalizations have all been adopted, or suggested, as possible measures for value-based pricing collaborations.
2. Confirm the pricing analysis
The second step in any pricing decision is to refine the analysis relative to the list prices of currently available products. These list prices act as price anchors, defining the value of new entrants in the market. In therapeutic areas that are already heavily genericized, companies must determine if the outcomes data they have are sufficient to enable reimbursement, and thus market share gains, given the existence of much cheaper therapeutic options.
Increasingly, stakeholders are willing to embrace “good enough” innovation if products satisfy basic safety and efficacy requirements but come with lower price tags. This is the value proposition associated with biosimilars and the second and third entrants in the all-oral hepatitis C category. Thus, companies need to understand that pricing flexibility occurs at only one specific time: when a drug is “only-in-class.” (See (Also see "The Shrinking Value Of Best-In-Class And First-In-Class Drugs" - In Vivo, 20 Jul, 2015.).)
That scenario obviously puts increased pressure on companies to deliver on their innovative pipelines. It also puts increased pressure on companies to embrace innovative pricing models. For instance, consider a new high-cost, but potentially high-impact product that is launching into a heavily genericized space, where there are “good enough” alternatives. To preserve as much flexibility as possible, companies in this situation could benefit from adopting innovative pricing strategies that allow them to collaborate with payers on the collection of outcomes data that accelerate the shift from potential to proven.
Novartis’ decision to pursue an innovative pricing strategy for Entresto provides important real-world context in this regard. Although the drug is first in class, its direct competitors include much cheaper angiotensin-converting enzyme inhibitors that provide “good enough” treatment for some percentage of CHF patients. But if Novartis is able to replicate in the real world the clinical trial data showing Entresto reduces expensive cardiac events, the downstream cost savings associated with reduced hospitalizations would offset its up-front price tag. This scenario makes the drug a good candidate for a novel pricing strategy. (An added bonus: the endpoint defining an improved outcome – reduced hospitalizations – could be easily measured using payers’ existing IT systems.)
3. Tie pricing to commercial strategy
The final step when articulating a product’s price is to link this decision to the overall business strategy, including the potential effect on the uptake of other medicines in the portfolio. For instance, the greater a product’s importance to a company’s overall portfolio, the greater the pressure to accelerate that product’s market share and close the value gap quickly. If there is significant stakeholder skepticism around a particular product attribute (for instance, time to outcome), a biopharma company might choose to adopt an innovative pricing strategy to bridge this particular value gap. In this instance, a novel pricing solution might be a means of co-creating additional data that are useful for demonstrating real-world value.
In addition, it is important that companies harmonize individual pricing decisions across the portfolio to create a coordinated commercial strategy. This step will become more important as more products are used in combination. Moreover, such a portfolio analysis enables companies to align portfolio decisions with overarching strategic choices, including decisions to invest in one business unit rather than another or the potential value creation that can come from divestitures.
The Road Ahead
The ongoing debate about drug pricing requires that, for their key products, biopharma companies embrace different pricing methods now, when the risks are lower and there is an opportunity to be an active partner in discussions with other stakeholders.
When drug pricing wasn’t as big a concern to other stakeholders, biopharma companies had the luxury of viewing alternative pricing mechanisms as a defensive option, reserved for use after negative value assessments resulted in market access delays that limited patient access. Going forward, however, companies need to understand that new pricing models enable access to valuable real-world data, the current currency of the reimbursement realm, and improve their reputations with other health care stakeholders.
EY believes that maintaining today’s pricing status quo comes with significant business risks. Current pricing practices already put biopharma companies in direct conflict with key stakeholders. Left unchanged, there is a real risk that payers will use blunt methods to curb costs, constraining revenue growth for the biopharmaceutical industry. More importantly, such tactics could limit patient access to vital therapies that improve the productivity and health of our global society.
Biopharma companies genuinely want to reorient stakeholder conversations to discuss the value drugs provide to patients and society. Those conversations will only be productive if biopharma companies first accept responsibility for developing drug pricing solutions that take into account stakeholders’ definitions of product value. Now is the time to think differently about drug pricing.
The views reflected in this article are the views of the authors and do not necessarily reflect the views of the global EY organization or its member firms.
Susan Garfield ([email protected]![]() ) is a Principal at EY specializing in market access and Ellen Licking ([email protected]) is a Senior Life Sciences Analyst at EY.
) is a Principal at EY specializing in market access and Ellen Licking ([email protected]) is a Senior Life Sciences Analyst at EY.
This article will be expanded in a forthcoming book co-written by Francoise Simon, PhD, Senior Faculty, Mount Sinai School of Medicine and Professor Emerita, Columbia Business School and EY's Glenn Giovannetti.
Acknowledgments
The authors would like to acknowledge the following individuals for providing assistance with the creation of this article: John Celentano, Russell Colton, David DeMarco, Patrick Flochel, Glen Giovannetti, Kim Ramko, Jeff Stoll and Gabriele Vanoli. Andrew Forman and Jasraj Sokhi developed the financial model presented in Exhibit 1.