Repositioning Market Access: A Function Fit For Purpose In A New Era Of Costly Cures
Executive Summary
On October 5, In Vivo convened a group of top market-access specialists and industry and investment analysts to consider a central strategic challenge facing all innovators in biopharma: how to pay for the next wave of cures. Finding the answer first depends on a rebranding of the function itself – it’s not market access to products; it’s patient access to progress.
- Industry must better understand and relate to each payer’s business model – especially those like integrated delivery networks and accountable care organizations that have a constituent interest in managing covered lives long enough to relate to the savings outcomes promised by industry innovations.
- More emphasis is required on nurturing in-house talent for a function with increasingly diverse applications while addressing a pervasive internal leadership gap – how much connectivity does market access have to the C-suite?
- So what? Those value-based drug evaluation frameworks are building roots and becoming institutionalized, with new precedents such as the partnership between the US Veterans Health Administration and the Institute for Clinical and Economic Review to use ICER research in selected VA drug formulary listing decisions. Proving value is no longer a voluntary exercise.
In addition to managing day-to-day relations with stakeholders, we focus on creative thinking about new ways to address rising health care costs and to make our medicines accessible to the patients most likely to benefit from them. What’s really vexing is managing the soaring cost of curative treatments. This is the next wave in health care. Its top of mind to us, and senior leaders expect answers.
So we have a big mandate, with more than 600 colleagues representing Patient and Health Impact throughout the world.
Of late, Pfizer has redoubled its efforts to improve ties to the customer. We follow a multi-channel communications strategy geared to making each of these constituencies understand our commitment to value. We are not comfortable with the notion that market access is an internal function requiring little interaction with the customer. Any group that has relevance to the therapies we bring forward is worthy of our attention. We will reach out.
Sudip Parikh, DIA: DIA is an association of industry, government and NGO regulatory and development specialists. I am senior vice president and director for the Americas region. Our focus is on the continuum of drug development, from basic research to postmarket activities in the commercial space. Until a few years ago, there was little talk in our meetings and publications about access and value issues – it was almost entirely centered on the R&D operation. Today, the situation has changed. One of the central questions for our 13,000 members is how their work fits in to this much bigger puzzle of how our products are sold and used by the patient, in the real-world clinical setting. Everyone is concerned about producing medicines in a way that expands coverage to patients who need them. Our initiatives reflect that interest. Programming at the DIA centers on strategies to increase collaboration between regulators, payers, providers and patients to identify value and deliver solutions that improve overall health outcomes.
In Vivo's Market Access Roundtable
James Coccia, Vice President, Oncology Market Access, Takeda Oncology, Takeda Pharmaceutical Co. Ltd.
Don Creighton, US Head, Market Access, ICON
Les Funtleyder, Health Care Portfolio Manager, E Squared Asset Management
Kyle Hvidsten, Vice President, HEOR and Value Assessment, Sanofi/Genzyme Pharmaceuticals
Julie Locklear, PharmD, Vice President, Health Economics and Outcomes Research, EMD Serono Inc.
Sudip Parikh, Senior Vice President and Managing Director, Americas, DIA Global
Amitabh Singh, PhD, Vice President, Patient and Health Impact, Pfizer Inc.
William Looney, Executive Editor, In Vivo, Informa Pharma Intelligence [Moderator]
James Coccia, Takeda Oncology: I am vice president for market access in the oncology business unit of Takeda. I began my career 20 years ago, when my function was called pricing and reimbursement. Our mandate in market access is simple to explain – but not simple to achieve. We work to minimize barriers for patients in obtaining therapy. It’s actually a very complex assignment, spanning everything from clinical development of the drug to commercialization, launch and postmarketing surveillance. And as our medicines become more personalized to the profile of each patient, it is necessary to provide additional support in the clinical setting. How to do that is top of mind for us.
External changes in how medicines are marketed and paid for has led to an upgrading of my role to senior management. A decade ago, the market access person did not have a place at the big table, fielding requests from a daunting array of internal and external stakeholders. It’s a dynamic, fast-changing environment. To be on top of the pricing situation two or three years hence, you must start preparing for it now. I’m sure we all agree it’s a big up-front investment in time and money to ensure our therapies get to the patient.
Kyle Hvidsten, Sanofi: I lead the health economics and value assessment group at Sanofi. We are one leg of what we call the market access tripod, with responsibility to generate the evidence identified by our product and pricing leads to demonstrate value to payers and other stakeholders. Sanofi aims to promote synergy among each part of the tripod. No one of us “owns” the value proposition we develop for each product, but the task of putting the arguments together is led by my market access colleagues. They build and coordinate the internal and external contacts needed to clarify the arguments and evidence that will resonate with payers, physicians and patients around the world.
Top of mind for me is grappling with a much bigger and varied group of stakeholders. The key stakeholder used to be the health care professional, but today the momentum has shifted to the third-party payer, who is less familiar with the real world of care delivery and would not be in the position to directly observe how a medicine is affecting patient lives.
What we see is a very public debate taking place, where opinion drives the discussion about price, affordability and value. This makes it more challenging to generate evidence from our product portfolio that is both objective and relatable to a more politicized environment. And it raises a larger question concerning how much weight health economics plays in determining the success of a new medicine. In fact, cost-effectiveness is harder to demonstrate for a medicine that is a true first-in-class breakthrough because there are few, if any, viable comparators.
Another issue we manage is integration of perspectives during the R&D process. It’s very important to present the full compound development plan to senior management. While we can all be laser-focused on what is valuable to our prospective customer, we still need to ensure that full perspective is incorporated when a development decision is taken. It’s critical that each component of the market access tripod makes the cut, but the sum has to be greater than its parts. Innovation that is sustainable within the existing delivery system for health care is an important consideration; this will come from more and better partnering relationships between the innovators and the those who deliver heath care services.
Les Funtleyder, E Squared Asset Management: I run the health care portfolio for E Squared. You might call us an unsung stakeholder. Our focus when we evaluate biopharma opportunities is the prospective price of the asset. It is not whether the price is high or low – we look for evidence of sustainability of the price in the competitive marketplace. We care about price volatility, which in turn relates to transparency. We want to know how the company and the key influencers like payers make decisions. Market pressures are less right now, if only because biopharma valuations are faring quite nicely and shares are up. The flip side is the various ways that government, regulators and the public are challenging the current industry pricing model. We see strong FDA interest in increasing competition in the industry by making it easier to apply to register complex generics, among other things. What’s top of mind right now? It’s clear that the government role in drug pricing is likely to grow over time.
Julie Locklear, EMD Serono: I will be leaving shortly a position where I led the health economics and outcomes research at EMD Serono Inc., a business of Merck KGAA in Darmstadt, Germany, for the last five years. I have been active in this field for more than 20 years. Much of that time has been spent championing the mission that a “value story” is relevant and necessary to position medicines for optimal use by patients, providers, payers and the overall health care system. As the pace of innovation has soared, so too has the interest and commitment by industry management.
My profession is also coping with a new set of stakeholders, who are referred to as “assessors.” These include the clinical guideline and pathway developers as well as the cost-effectiveness evaluation groups, most of whom did not exist five years ago. Employers are also emerging as a key stakeholder, due to their increased interest in proactively managing their cost exposures, especially regarding chronic illness disabilities that impair the productivity of the workforce.
My function is now located in the medical affairs space at EMD Serono, after many years being located in the commercial business with managed market responsibilities. As a result, the team is much more centered on building a value proposition that works for patients first. My team works with managed markets and since we’ve been in Medical Affairs, we also focus on scientific leadership by publishing real-world effectiveness research. That is what’s top of mind for us today.
Don Creighton, ICON: ICON has a new division called Commercialization and Outcomes, incorporating real-world evidence, pricing, market access, health economics, medical affairs and diagnostic and device research. The upgrade is representative of a broader, industry-wide trend in seeking to gain a better grasp of the complex interactions involved in getting a new medicine into the hands of providers and patients. I think we’d agree that the process is imperfect – true integration of the relevant capabilities around market access remains a work in progress.
Nevertheless, our own work with companies reveals that market access is now seen as a strategic driver across the organization. It’s no longer a narrow technical function embodied in the old P&R model, which was an add-on activity, usually reporting in to corporate finance rather than the commercial business, and whose contributions occurred at the very end of the development to registration cycle. There is also a stronger outward-looking emphasis linked to the importance the concept of “value” now plays in determining when, where and how a new product gets introduced to patients. A value argument can be constructed internally, but it must be verified – and accepted – externally, in the market. And as the importance of this exposure grows, it is possible market access will be designated as a standard bearer at the C-suite level – Chief Value Officer (CVO) comes to mind.
Is this an upgrade in responsibility that we can endorse? We think it holds promise, if only because market access is increasingly a messaging and public relations exercise and you need a person with visibility and clout to do that.
Another top-of-mind concern is finding the right talent in market access. To meet the demands of payers who control access to millions of covered patient lives, mere technical competence is not enough. That must be combined with “soft” skills like communications and the other arts of persuasion, a strong external contact network, and the ability to lead and build consensus in teams. How do organizations identify and attract people with both the technical competence and the interpersonal and leader behaviors?
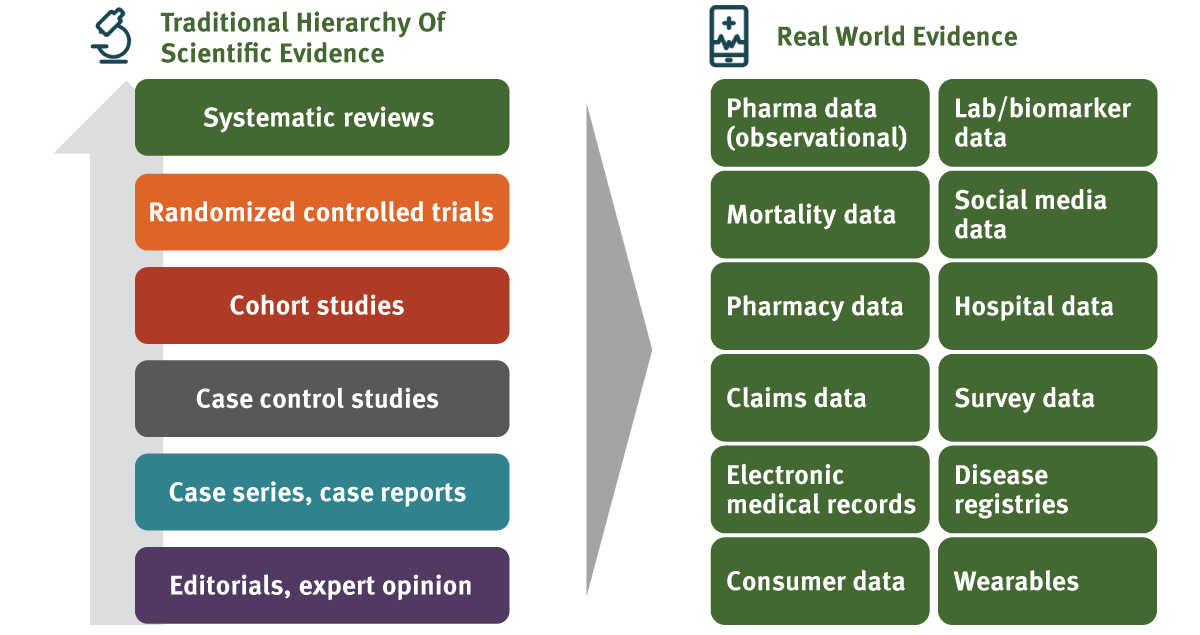
And then we come to the issue of evidence. It is no longer possible to launch a new medicine without a robust, data-driven value proposition in hand. Making the challenge even harder is the proliferation of data and the knowledge and insights that flow from it (see Exhibit 1). How do you sift through all this information and break it down in a format relatable to that payer guardian of the patient? How do you combine the best clinical information about the product with the appropriate financial assessment linked to the price?
It’s equally important to break down the motivations of the various stakeholders. There is a single-minded emphasis on the payer, but outreach to providers and patients can determine success as well. Surprisingly, we find that companies fall short in communicating their value message consistently, to all comers. Insufficient attention is paid to building the value message when detailing to physicians or through the medical affairs liaison.
Pricing, and its underlying rationale, is often not even addressed at the time of product launch, which leaves companies exposed when other actors, like the media, try to fill that gap with their own back-of-the-envelope calculations. Much of the commentary is woefully ignorant. For example, we often see misrepresentation of basic approaches to fixing a price. For example, the media will take a monthly price and multiply it by 12 to determine the cost to the patient; however, the median duration of treatment may be much less than a year – and that is never factored in. The rise of value “frameworks,” and independently funded drug evaluation bodies like ICER, each with a different rationale and agenda, is due to the failure of industry to be more transparent about the price argument as it is on issues of safety and efficacy. It is necessary to extend the conversation about price and value to many more parties with interest in a price transaction – but does the industry have the will to roll with the punches and do it?
Exhibit 1
Prioritization Of Evidence Generation
ICON
Singh: All of us want to find some common ground with key emerging players like the Institute for Clinical and Economic Review (ICER). Pfizer regularly gives them feedback on their therapeutic surveys and reports that are relevant to our business.
Our group established four core principles we think will help structure the process. These are: patient centricity, by incorporating patient-reported outcomes and other relevant evidence in ICER reviews; a multi-dimensional approach to evidence, one that supplements narrow criteria like the quality adjusted life year (QALY); allowance for assessment of benefit over time; and, most important, making the determination of cost-effectiveness fully transparent and open to debate. We think this latter principle represents an area where these organizations could do a lot more in concert with industry.
Locklear: All of us in industry would agree with these four principles. They are ambitious; the importance attributed to a generous time horizon in establishing whether a treatment delivers value to patients and the health system is vital. Yet it runs counter to the status quo budget cycle that gives no credit to therapies that improve overall health outcomes three or more years down the road. These are structural challenges within the health system that cannot be resolved in the course of a methodological exercise focused on a single drug. In fact, medicines overall account for only a little more than a tenth of total annual spending on health in the US.
Hvidsten: It is equally true that the horizon for establishing value is limited by what is feasible to demonstrate in the standard randomized clinical trial. That leaves modeling exercises as a feasible option, but they are scenario-based and cannot yield the data-based evidence to prove value over time.
I also want to emphasize that the sponsors of these value frameworks are genuinely open to discussing ways to incorporate input from the patient. The question everyone is struggling with is – what do they do with it? There is an inherent conflict between evidence, based on patient anecdotes, and the quantitative methodologies that data scientists rely on to evaluate whether or not a technology is cost-effective.
Singh: One question we ponder is – why it has been so slow to bring patients fully into the drug development process. Every clinical study here in the US must obtain the “informed consent” of every participant we recruit. Why can’t we take that a further step, in concert with the FDA, in bringing patients in to review the trial protocol and comment on the relevance of study endpoints in terms of what patients with the disease are actually experiencing? Pfizer also has a strategic focus on rare disease, where recruitment of subjects can be challenging. Working with patient representatives can be invaluable in reaching the appropriate people.
Coccia: Takeda Oncology just started a longitudinal trial with 5,000 enlisted patients where we have put a patient representative on the trial steering committee. It’s a complex trial but we now have a single, high-level focal point to ensure that patient perspective follows right through to the end.
Hvidsten: Patients are central to Sanofi’s stakeholder engagement strategy. I ask every member of my team with therapeutic area responsibilities to liaise directly, as appropriate, with patients. I want to make sure we hear their stories and incorporate these into the materials we develop around value.
Creighton: This is a very positive sign from an internal perspective, but in my view industry is still not doing an adequate job in articulating a medicine’s value to the patient – the momentum still rests with evidence that responds to payers and providers. Due to industry-led contributions to advances in oncology, the median overall survival rates for cancers like multiple myeloma and non-small cell lung cancer can now be measured in years instead of months. That’s a near miraculous turnaround for patients. Yet it rarely gets raised in the conversation with organizations like ICER, which tend to focus on high-priced drugs for malignancies that are hardest to treat. They don’t understand progress is cumulative around seemingly small steps forward. Indeed, if we were all doing so well in communicating the value industry brings, we would not be having this discussion today.
Parikh: Trade association efforts to communicate the value of new medicines are well funded but the messaging usually seems to fall short. PhRMA’s current “Go Boldly” campaign has been described to me as tone deaf in not reaching out to the partnering institutions that are often crucial in establishing the science behind the drug. That’s important because the public is rightly skeptical if the advocacy is only funded by the drug industry.
Singh: Industry fails to prioritize around its messaging on value. First and foremost, we must highlight the impact of innovation in medicine on the individual patient. Next, explain what medicines are doing to help the overall system of health care cope with its problems. And finally, what the current incentives to invest in innovation mean for mitigating the big risk in discovering the next generation of transformative medicines. These are the three communication “guardrails” to steer the debate and make our case more effectively.
Coccia: It is often true that people close to us – our parents and families – lack any real understanding of what we do. We have to change the way the outside world thinks of this industry. The first step is to explain that our focus is on therapies, not just the product.
Creighton: I suspect Novartis is very confident that as a platform technology this will lead to new indications in areas beyond oncology, such as rheumatoid arthritis, Crohn’s disease and other auto-immune conditions. You can’t rule out the prospect for multiple, billion-dollar plus returns from CAR-T. And cell therapy is basically a one-time treatment that results in a cure. Hence, the cost exposure to all these ancillary services can be contained, while efficiencies should increase under real-world clinical use.
Hvidsten: The constructive position for the industry is to stress that we must always go where the science takes us, even if the result is a complex pricing model that tries to reconcile the cost of investment with the pressure for access. The access discussion also must relate to the need to maintain the momentum for innovation – to fund those future refinements to therapy that will benefit patients. It will always be difficult for the company that leads first with a genuine breakthrough. But the difficulty lessens as follow-on products are developed and come on stream.
Singh: Agreed. But I would amend the calculation slightly by emphasizing that the science must be focused on where there is the greatest unmet need. That is, the science should coincide with the societal interest.
Parikh: The politics around pricing are becoming more onerous. Twenty years ago, we talked about the “valley of death” as what happens when a molecule fails to progress from proof-of-concept to an actual human trial. Today, the valley of death hinges on perceptions about the access model – the economics of getting the medicine to the patient and sufficient dollars back to the manufacturer to ensure a return on their investment. The money might be there for development. The challenge is it may not be there for access to the covered patient.
Locklear: The real “valley of death” occurs when society yields to the view that a new medicine isn’t worth the benefit in terms of giving people longer, healthier and productive lives. Unfortunately, we are not having much success in persuading more stakeholders to enter a very public discussion on the implications of this view. It’s also disappointing that payers and regulators still tend to resist a “patient-centric” approach to value, which can only come when the patient experience is included in pricing and access decisions. To understand the patient you first must know the patient.
Coccia: Our industry tends to associate innovation with the ability to charge more. The presumption is that if you are adding a benefit over existing therapy, then it deserves some premium. Takeda has a product that was reviewed under the MSK Drug Abacus framework, which actually found it to be underpriced in comparison to existing therapy. This created a dilemma when we finally got our next-generation medicine approved. We struggled with the idea of asking payers and patients to choose between staying with the standard therapy or moving to the new product at a higher price point. It can be a difficult conversation – asking the same group of patients to pay more – with a lot at stake for the business if we get it wrong. Ultimately, we priced the new product on par with its predecessor.
Creighton: Pricing to the market is always a judgment call. That represents a special challenge to an industry so heavily scrutinized. How do you justify a price for a product that represents an “incremental” innovation? Should the customer – in pharma, that usually means a third party who will not be taking the drug directly – decide what that means? Does the product deserve a 50% premium over existing therapy? Or a 20% premium instead? Is the evidence clear enough to evaluate the cost using some standard metric like overall survival rates? If so, how does the manufacturer communicate that consistently, with clarity, to stakeholders with different commercial interests? There is no formula that works for every case. That’s why we say market access is a mix of the technical and the artistic.
Singh: We must acknowledge that the health care system is moving from a utilization model to an impact model. There is less middle ground to accept something incremental that could also be called “me too.” Society is no longer willing to pay for anything less than an upgrade in the standard of care. If the data doesn’t show that, the price conversation won’t yield anything fruitful.
Coccia: The era of double-digit price increases for existing medicines is over. This year, most big pharma companies have pledged – voluntarily – not to do that.
"Pricing to the market is always a judgment call… There is no formula that works for every case. That’s why we say market access is a mix of the technical and the artistic." – Don Creighton, ICON
Hvidsten: Not only is there a discussion, the process now in use to get approval for an expensive medicine applies to everyone – it’s become more uniform and less tailored to the individual circumstance.
Creighton: Despite the aggressive stance of many payers, the US continues to require open access to some products under the Medicare Part D benefit. That makes the private sector a bit more reluctant to buck the precedent and bar coverage. You also see how clinical pathways in oncology rely heavily on peer-reviewed physician assessment, which gives the payer some cover in decisions on access and reimbursement. The payer doesn’t really want to own the problem alone.
Coccia: The Center for Medicare and Medicaid Innovation (CMMI), also part of CMS, wrote an op ed in The Wall Street Journal a few weeks ago that basically asked for ideas on how to better manage their drugs bill. I agree there is more to come from the payer side, and, from our point of view, relying on clinical pathways written with the assent of top physicians in clinical practice is probably the simplest way to put pressure on us – while avoiding being tagged as the messenger of that absolute “no” to the patients. It’s more of a carrot than a stick.
Funtleyder: Our health care system is highly competitive, with different segments all seeking to take as much as they can out of the budget pie. Drugmakers need to be part of the conversation as these allocational challenges are worked out among private commercial plans and in the federal bureaucracy. You need to show all these interested parties that your product will help them save money. That task is critical as you face pressure to make your own costs more transparent. Other groups – like hospitals – will be all too willing to force you to do that, especially if it deflects attention from their own behaviors. Hospital care accounts for by far the largest proportion of US health spending, but it is rare to find any prominent politician willing to state “hospitals charge too much for what they deliver.”
Singh: The value model is slowly gaining traction as the preferred alternative to traditional fee-for-service. The federal government is mandating the transition in Medicare through legislation that forces providers and hospitals to observe quality-of-care measures and real-world outcomes to get paid. About one-third of Medicare payments are now filtering through this value-driven model, and the aim is to push that much higher in the next two years. Likewise, the big commercial payers estimate about 40%of payments to physicians are now value-based.
There are also the IDNs and accountable care organizations (ACOs) that are incentivized under the Affordable Care Act (ACA) to contract with drug companies around measures that link formulary access to outcomes from treatment. Both are motivated to consider the savings from drug therapy because they retain covered lives for a much longer period than is the case with traditional insurers or the PBMs that contract with employers.
Creighton: Co-pay assistance and co-insurance are the principal tools being used today. Most companies have active programs, administered on their own or through a non-profit independent foundation. I expect companies to focus on these programs rather than entering the financing and insurance business directly.
Coccia: Co-pay assistance is good but awareness among the patient population is quite low. That has to change.
Singh: Pfizer has programs in place to help patients access our drugs at an affordable cost. An example is the RxPathway program. Last year, we had a quarter of a million patients receiving Pfizer medicines through RxPathway, and two million prescriptions were filled. Nevertheless, I don’t believe there can be just one solution to facilitate access. It is in the industry’s interest to try different approaches, including those “out of the box” ideas like annuities or bonds or a social contract in partnership with government or private philanthropy. Let’s see what thrives from the diversity.
Parikh: The industry associations also coordinate such programs. But few people know about them. And there is a fundamental flaw at the heart of any co-pay assistance outreach. You don’t hit people when it’s time for them to pay. I’d also offer that the “out of the box” alternatives are too complex. You get all those confusing externalities that economists like to talk about.
Funtleyder: I wonder why PhRMA has been so slow to embrace a multi-channel marketing approach to create more awareness of the industry contribution. Why not do a YouTube 10-minute video that involves some clever use of pictures and audio to explain what CAR-T therapy can do for the patient and the future of medicine? The value is that it is easily accessible, very inexpensive to produce and is not product specific. It is a teachable moment on a ubiquitous platform.
Singh: Nevertheless, we must consider the impression such a video might give to patients in need of treatment. An ambitious disease awareness program can be preferable in relating directly to patients without assuming to direct their health care.
Locklear: But it’s worth emulating what Les said about the importance of a multi-channel communications strategy. We are missing too many connections with a changing health care demographic, including many younger people who are very comfortable in interacting with health providers remotely rather than in person.
"There is a fundamental flaw at the heart of any co-pay assistance outreach. You don’t hit people when it’s time for them to pay." - Sudip Parikh, DIA
Funtleyder: Every start-up knows that if there are no slides in the pitch deck explaining how it will convince the payer there is a feasible market demand, they won’t get any money. It’s inevitable the person with this role is going to move higher in the corporate food chain.
Coccia: Getting a new medicine to the market is built on a stool with three legs – the commercial organization, medical affairs and market access, which we might also reference as the value piece of it. All three must work together to ensure success. We know that problems often occur when we rely too much on one or two of the stools. It may be that designating a CVO position in the C-suite could end the situation when that third leg is missing.
Singh: A CVO could indeed be helpful. But the real issue is this: has your company put someone in charge of market access with the standing to pick up the phone and speak directly to the CEO?
Locklear: You also need a group with the capabilities and skill to educate the entire senior leadership on the access landscape. Literally, the goal is to equip them to ask the right questions.
Coccia: The best way for the market access team to exert influence is to be a key contributor to the initial product forecasting plan. If market access gets cut off from that process, then you are in trouble – because the numbers built in to the plan are tracked constantly.
Hvidsten: It’s also important that market access accentuate its position as a liaison to the ultimate beneficiary of a new medicine: the patient.
Funtleyder: Exactly. In fact, I propose the industry dispense with the title “market access” and change it to “patient benefit.” Market access indicates the mission is really one of building sales and winning customers, none of whom are patients. It looks as if you are just trying to sell me something. Likewise, I think the entire notion of “value” is too amorphous to relate to an individual therapy. It’s like a Jeff Koons sculpture – What, that bunny costs how much? Why?
Coccia: Our approach is to put most of the emphasis on the access part. I agree that changing the lexicon is important. Every time we refer to medicine as a “product,” I cringe. Cars and candy are products. We develop therapies for people who are suffering from disease.
Coccia: It’s harder than ever to fill those open slots on market access. We are finding a solution in working to develop more people for these roles internally.
"The challenge is finding talent able to navigate through all these strands – being familiar with the science, the numbers and the evidence metrics; having strong people skills and an awareness of the policy dynamics outside the office; all accompanied by the ability to synthesize from experience and present the big picture. It’s a challenge." – Amitabh Singh, Pfizer
On the other hand, I think our R&D people are moving the ball forward in applying technologies like AI to help our cell biologists and chemical engineers identify new platforms for drug discovery and delivery. The key objective here is relating the new technology to improving patient care.
Hvidsten: New technologies that advance our understanding of real-world outcomes might help clarify and de-risk the negotiation of more value-based contracts.
Locklear: We haven’t spoken much about the impact of real-world evidence (RWE). Our understanding of cloud-based technologies, advanced analytics, machine learning and AI is growing. There are real gains to be had from this, shortening the time line from discovery to regulatory approval through efficiencies in the clinical trial process. If we agree that market access is really patient access, just pitch your colleagues in R&D on how to use RWE to solve the problem of patient trial recruitment. Your function suddenly becomes immensely important to them. Just follow the data. And that data – the real-world evidence – will also help in creating, executing and monitoring risk-sharing or value-based contracts where confirmatory evidence is critical to validating any successful partnership.
Funtleyder: The era of drug delivery by drones and zipline has already arrived in countries like Rwanda, where these new technologies are filling the gap left by the lack of traditional supply chain logistics and infrastructure. Amazon has an internal group working on ways to enter what it believes to be a hidebound sector ripe for disruption, especially by entrants willing to be a loss leader to create market share. Medical devices – an area where Amazon has some prior exposure – could be a viable option rather than drugs.
Parikh: There are significant barriers to entry – health care is a complex business where there is a fissure between who pays for a drug and who uses it. Amazon usually works in fields where there are no regulators to complicate interactions with the customer. Government regulation of those interactions is extensive and legally fraught. Hence, the idea that Amazon can roll in and start developing drugs for consumers is a bit optimistic. The consensus is the company will be highly selective on where it intends to engage – most likely as a PBM, distributor or pharmacy – I think it almost certainly won’t be developing and marketing medicines.
Coccia: As long as there are questions about patients getting access to the drugs they need, our role in the business is justified. Nevertheless, we highlighted today the time is right to develop the next iteration of the market access function. It must be much more patient-centric than it has been to date. We have to double down around the goal of thinking of the patient perspective – every day.
Singh: Our most fundamental activities will not change over the next five years. These are three: (1) providing strategic direction to the business, and executing around it; (2) facilitating cross-functional, geographic and external stakeholder collaborations; and (3) building and replenishing market access capabilities in all parts of the organization. Much of the emphasis is going to fall in the second bucket, because a premium will be placed on finding sustainable ways to keep a rising new class of curative medicines affordable. We will need to be forward-looking innovators rather than the old “block and tackle” group that deflects problems rather than solves them.
Locklear: I am optimistic that market access will be seen as integral to the solutions approach to health care, where no company will be comfortable in just punching out pills. The challenge is going to be keeping everyone – including the commercial team – focused on improving the patient outcome.
Parikh: I believe you are almost there in being considered as part of the team by the R&D business. I can’t envision any scenario where market access will not be part of a go/no-go decision on bringing new compounds forward to patients. That’s a good place to be.
Creighton: I worry that people in the market access space are going to feel stretched over the next five years. Expectations from management about what this function should do are high. That’s what happens when any capability gets more exposure in the organization. And recruiting people with the right skills and keeping people on board is already a challenge. Just consider the implications of the increasing number of external players who want to restrict, not widen, access to new innovative medicines. Payers’ pricing power is consolidating, while the IDNs and other new system providers are demanding more than a standard sales contract from drug manufacturers. It’s a combative front line of engagement, and it’s expanding. We will have to respond proactively to this new dynamic, while also doing our day jobs.