Building A Biotech: Industry Veteran Moroney Reflects
Executive Summary
Simon Moroney has led the German antibody technology biotech MorphoSys since 1992. Having taken the decision to retire just as the firm is entering the next phase in its evolution, he explains to In Vivo why the time is right.
Simon Moroney, co-founder and CEO of Munich-based biotech MorphoSys AG has made clear his plans to retire by June 2020. He has been at the helm of MorphoSys since its inception 27 years ago, and believes that the company has now reached a turning point, becoming more competitive and controlling its own destiny, which creates an opportune point to hand the reins to someone that can lead the company through its next stage of development.
In an exclusive interview, New Zealand-born Moroney said he felt it was vital to ensure the company was in great shape before he took the decision to leave. “It’s in a really strong position; its prospects have never been better. So it feels totally like the right time for me and for the company: from that perspective I feel very good about it.”
The past three years have seen the biotech make moves that will catalyze the next stage of its growth. MorphoSys licensee Janssen Biotech Inc. gained approval in the US and Europe for the plaque psoriasis drug Tremfya (guselkumab), to which MorphoSys will be entitled to royalties; it filed an IPO on the NASDAQ to garner US investment; and it received FDA breakthrough therapy designation for MOR208, its proprietary lymphoma antibody for relapsed or refractory diffuse large B cell lymphoma.
"I would never have imagined at the outset that we could have been so productive." - Simon Moroney, CEO of MorphoSys
After years of partnering with big pharma such as Roche and Novartis AG, MorphoSys is to commercialize MOR208 itself, potentially from the middle of 2020. This, coupled with the appointment of David Trexler, ex SVP of EMD Serono Inc.’s US oncology commercial division, as the new president of the company’s recently founded US subsidiary, MorphoSys US Inc., show a company gearing up to be commercially more assertive.
“We’d be in the market, up against much bigger companies than us, much more experienced commercial companies, so we understand the magnitude of that challenge,” said Moroney. “We think we’re up for it. There are multiple examples of American companies, mainly, that have done this themselves, so we’re not trying to do anything totally new here, but it will be a new step in the company’s development, there’s no question. We’re preparing for it very actively."
There will be a rolling submission during the course of this year to the FDA for MOR208, which will complete by the end of 2019. MorphoSys is hoping for approval by around mid-2020.
Moroney will remain a shareholder and says he is “super excited about what’s coming along,” but concedes that he doesn’t need to be CEO for the next stage of the company’s evolution.
While the commercialization of MOR208 will potentially coincide with his departure, Moroney will retain a strong vested interest in the success of the drug, and the rest of the blossoming pipeline. The company currently has more than 100 programs in total, with 29 of those in clinical development.
“I’m not aware of any other single platform in the industry that has been so productive,” said Moroney. “We’re talking here about a single platform. It helps that we’re in an area – antibodies – which can be widely applied across multiple indications. I would never have imagined at the outset that we could have been so productive.”
A Burgeoning Concern
The outset that Moroney refers back to was in 1992. He had been working in academic positions at institutions including Cambridge University and Harvard Medical School but had had a taste of industry with a stint at ImmunoGen Inc. working on the first generation of anticancer antibody conjugates.
Having realized that publishing and teaching did not hold his interest as they once had, and with the “challenge of building something significant” turning his head, Moroney and his colleague Christian Schneider formed the idea to build a platform technology company.

The pair then approached Andreas Plückthun, an antibody engineer who had made several important breakthroughs in the field. With Plückthun on board, and his technology forming the nucleus of the company, MorphoSys started out with €150,000 in venture capital funding and a pretty big challenge; to build a collection of over one billion different human antibodies as a basis for the development of new drugs.
“We started it as an absolutely trainee start-up with nothing and almost no money and so developing our own drugs to any extent was just not realistic at that time,” said Moroney. In the early 1990s, the platform technology business model was en vogue, he recalled. “At the time, it was frowned upon to try and develop drugs yourself. That sort of heritage is visible in our incredibly broad pipeline, which is so broad because we worked with so many different pharmaceutical companies in the early days.”
Today, the company’s partnership roster is an A-Z of the pharmaceutical industry. Names such as Pfizer Inc., Merck KGAA, GlaxoSmithKline PLC and Bayer AG all make an appearance, using MorphoSys proprietary platforms HuCAL and Ylanthia to discover and develop antibodies.
"I hope that MorphoSys has acted as a kind of beacon, a lighthouse if you like, of what other European biotech companies can be like.”
In the past 27 years the company has changed organically. Essentially starting out as a services business it transformed into a development business which required a big change within the company, both in culture, personnel and in-house expertise, see timeline below. “Now we’re standing at the beginning of the next change,” said Moroney, adding that the company's focus is on its proprietary programs.
With the long-standing CEO's departure the biggest shift in the company’s evolution to date, and by treading in commercialization territory, it will have to toughen its shell to the highs and lows of a globally competitive industry.
Recent Setbacks
A fresh example is the January US court ruling against MorphoSys in its three-year patent dispute with Danish biotech Genmab AS and pharma giant Johnson & Johnson over Darzalex (daratumumab). (Also see "Shaken MorphoSys ‘Assessing Options’ After Losing US Darzalex Patent Ruling " - Scrip, 28 Jan, 2019.) Moroney appears surprisingly relaxed when he comments on the patent decision. "Of course we invested in the protection of our IP, but in the end, a court case case is always a bit like a lottery ticket."
“It was disappointing, obviously. We would have love to have prevailed; we would love to have a royalty on Darzalex. But it wasn’t to be and honestly, it doesn’t really matter for us. We still have patent protection on MOR202, which is the key protection we need.”
In the ruling three US patents were ruled invalid, those patents were only important to MorphoSys in that they may have covered Darzalex and therefore, earned the German biotech a royalty on the blood cancer blockbuster.
Moroney said the company was “caught out a little bit” by a “real tectonic shift in the way patent law is interpreted in the US,” which will affect other companies. “That was something that we couldn’t have expected at the time that we launched the suit and it just turned out that way; it’s unfortunate,” he said.
Onwards And Upwards
Moroney is undecided in his next move. He feels he could be helpful as an advisor to European biotechs, but has no solid plans, and will not be starting a new company.
Through his 27 years in the European biotech community Moroney has witnessed many a biotech boom and bust. The lack of ambition and belief within smaller European biotechs is sometimes a worry, he said, and he hopes that MorphoSys can inspire smaller companies to be competitive on a global scale. “I hope that MorphoSys has acted as a kind of beacon, a lighthouse if you like, of what other European biotech companies can be like.”
Moroney’s message to inspire small European companies is to be confident: “You don’t have to give your product away early; you can go out and raise sizable amounts of money to do the development yourself, to hang onto the rights yourself.”
He regrets that there aren’t more large European biotechs, but cites the success of companies such as Galapagos NV, Genmab and Evotec AG as examples to smaller companies to develop drugs themselves, which “is the real way of creating value in this industry.”
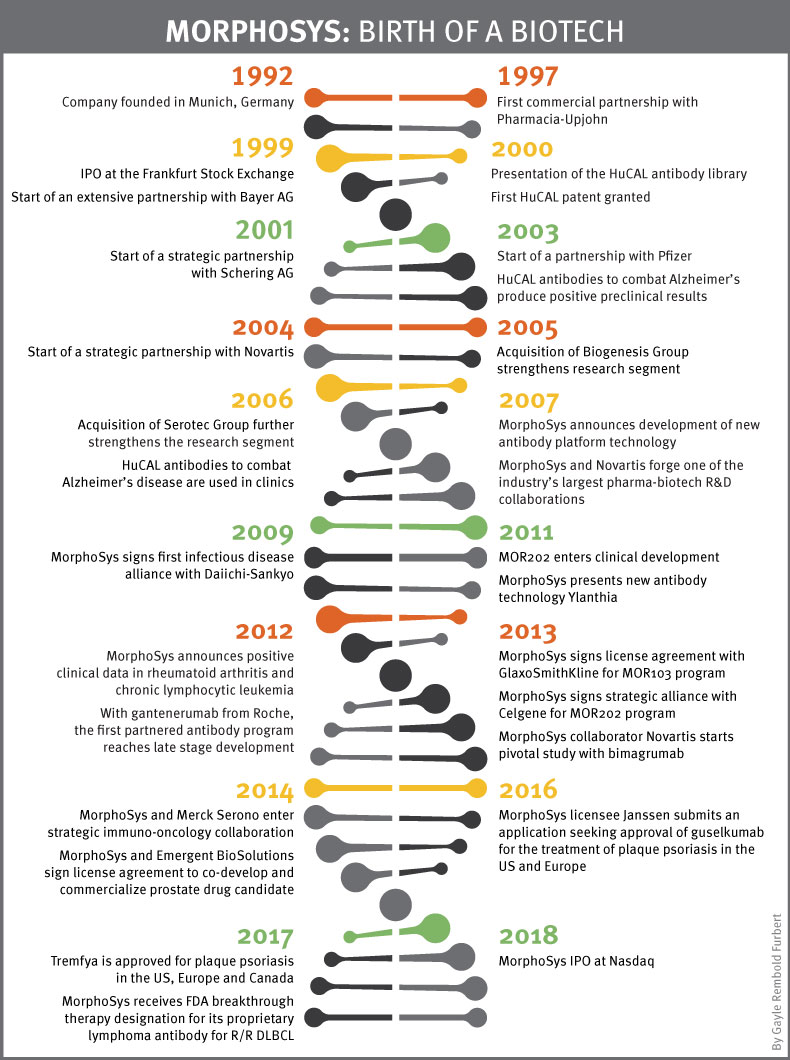
Download PDF version of timeline
[Editor's note: This article first appeared in Scrip, In Vivo's sister publication.]
