Unlocking Resiliency In Clinical Research And Improving Access For All
Executive Summary
The pharma industry has grappled for years with its role in improving equitable access to clinical research. The needle has moved, but impactful changes have not come quickly enough.
Clinical research is the key to developing and evaluating innovative treatments, yet marginalized communities continue to be under-represented in clinical trials. Without diverse participation that reflects the broader population, the full scope of potential risks and benefits of a product cannot be fully understood by developers. In addition, the exclusion from clinical research can deprive these communities from access to cutting-edge, potentially life-saving treatments, and perpetuate existing inequalities in health care.
The FDA Center for Drug Evaluation and Research publishes an annual Drug Trials Snapshot capturing the diversity of participants in trials for approved novel drugs that year. In 2019, across 48 newly approved drugs from studies that included more than 46,000 participants, only 9% of participants identified as African American or Black, only 9% identified as Asian, and only 18% identified as Hispanic. Many marginalized communities suffer the consequences of exclusion from clinical research; racial and ethnic minorities, LGBTQ+ individuals, people over 65 years of age, and women are often under-represented. Exclusion has significant consequences that can resonate across the health care community.
Examples demonstrating this issue:
-
Race: African Americans have 2x the incidence of multiple myeloma as White Americas, accounting for 20% of the currently diagnosed multiple myeloma patient population, and yet, account for only 6% of participants enrolled in multiple myeloma clinical trials. There are known differences in the cancer genetics of African Americans and White Americans, putting at risk the opportunities to identify new therapies that adequately address this population.
-
Gender: Research has shown that women are at a 1.5-1.7x greater risk of experiencing adverse drug reactions. This represents hundreds of millions of dollars in associated health care expenses for treating those drug reactions. Accenture Research (using FDA information alongside data collected by HEOR and biotech companies) calculated the average cost of treating adverse events from oncology products across the most prevalent types of cancer. This research indicated that adverse drug reactions could account for an additional $780m-$825m in health care expenses.
-
Minority Groups: Use of mobile tech can improve the representation of sexual and gender minorities (SGM) in clinical research. The 2019 "PRIDE" (Population Research in Identity and Disparities for Equality) study enabled SGM people to provide demographic and health data. Of more than 16,000 participants, more than 98% identified as a sexual minority, and more than 15% identified as a gender minority.
There is no single solution for the problem of clinical under-representation; the barriers to inclusion vary for demographic, economic, geographic, and historic reasons. For example, from an economic perspective, patients who do not attend regular medical visits because they cannot afford to also cannot be referred into clinical research by the traditional methods of recruitment due to prohibitive out of pocket related expenses.
From a geographic perspective, patients from marginalized communities are impacted by their proximity to research centers – the distance from a clinical research site or academic medical center is often a significant burden on patients. According to Sean Lynch, director of study operations at TrialSpark, half of FDA trials in the US occur within 1-2% of zipcodes. It is critical to note, these systemic issues are amplified by mistrust of the US health system due to historic and, sadly, contemporary abuse from the health care community on marginalized communities. A seismic shift is needed to address these barriers and to increase access for marginalized communities.
Revelations From COVID-19
The realities of the COVID-19 pandemic are now a burning platform for addressing equitable clinical representation and underscored the importance of a deliberate effort to transform our traditional clinical research approaches. In the US, people of color (including African Americans, Native Americans and Hispanic Americans) have suffered greater risk of contracting, being hospitalized by, and dying from COVID-19, ranging from a scale of 3-4x greater risk of being hospitalized and dying from COVID-19 than White communities.
Additionally, the pandemic has laid bare the inadequacies of having a fully centralized clinical trial strategy (i.e., dependent on the traditional site-based model) and essentially forced an overnight shift to adopting some digital solutions. Nearly 50% of clinical trial sites stated that COVID-19 had impacted their ability to start new trials, with approximately 31% of sites reporting delaying their trials and 14% reporting cancelling trials altogether. A growing majority of clinical sites have scrambled to re-evaluate their study operations, with approximately 40% shifting to virtual or remote patient visits. The industry needs to find a path forward that provides resiliency, and it cannot do so while actively excluding whole swaths of the population. The implementation of patient-centric clinical trial design and digital solutions that account for the specific needs across communities can contribute to improving the efficacy of the product and the ultimate success of that product – while driving more equitable access for the marginalized patient population.
It is highly appropriate to evaluate the success that Pfizer Inc., Moderna, Inc. and other COVID-19 vaccine manufacturers have had with enrolling diverse populations. While no company has reached the gold standard achieving parity with census numbers, they managed to beat the clinical trial average and stay in line with the 2019 averages for new drug applications and biologics license applications. And all while operationalizing in record times.
Moderna Case Study
With the race on to develop a suite of vaccines across manufacturers, Moderna took meaningful steps to ensure late-stage development for its vaccine included significant representation of the communities most at risk during the pandemic, actually slowing their trial timeline. Moderna CEO Stephane Bancel said, “I would rather we have higher diverse participants and take one extra week … [Diversity] matters more to us than speed.”
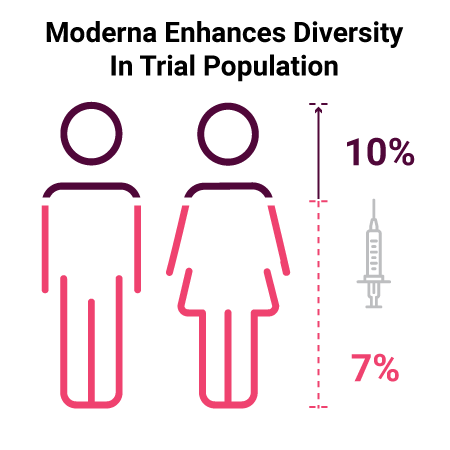
Moderna changed recruitment and study operations in a way that allowed them to enhance the diversity of their participant population. They shifted away from the traditional model of recruitment and utilized a massive online registration process and targeted specific enrollment sites to meet their goals. Within just three weeks, the enrollment of Black patients within their Phase III trial grew from 7% to 10%.
Moving beyond clinical recruitment and enrolment, Moderna also stood up a new vision of patient engagement to alleviate the burden of participation on these patients and improve long-term retention in the study. Instead of the often burdensome trial design requiring patients to frequently make in-person visits to the clinical trial site, patients were surveyed remotely via tele-consults and e-Diary logs for the duration of the study, and even patients with COVID-19 symptoms could opt for either in-person visits at their convenience or receive a medical consultation in their home. For patients, this can be the difference between finishing a study or dropping out early due to the expense of transportation and childcare, time away from work or dependents, and other significant inconveniences that often disproportionality affect marginalized communities.
The success Moderna and other manufacturers have had in developing the COVID-19 vaccines provides lessons to take forward as we redefine best practices for clinical trial recruitment. The industry can prioritize equitable representation to improve how it pursues recruitment, enrolment and handles retention. Moving forward from this pandemic there are opportunities to revolutionize the way clinical research is conducted. Research can be more resilient while simultaneously serving a broader population of patients.
Improving Trial Diversity
To better understand tactical actions the industry may take to improve diversity and access in clinical trials, Accenture research analyses were conducted on the full venture and mature landscape of clinical trial offerings. Remarkably, out of more than 1,100 individual companies creating assets and solutions for this category, less than 19% proposed to deliver impact on outcomes that would affect underrepresentation. Even fewer actively address diversity and access as a service benefit.
Industry action is slowly taking root: PhRMA announced the first industry-wide principles for diversity in clinical trials in November 2020, which will take effect in April 2021. It pledges to address health equity in underserved populations, including racial inequality. Individual companies have programs in place to have trials represent the composition of patient population. For instance, Eli Lilly and Company, Johnson & Johnson and Pfizer are seeking to improve participation of diverse populations, in some cases providing transportation assistance to trial sites, and improving diversity in site staff and investigators who provide the care.
The aftermath of government shut-downs and human isolation has boosted the virtual and decentralized clinical industry. Across more established entities, Accenture estimates that between 300-500 trials have virtual components, and that these numbers are likely to triple in the next 3 years. In discussions with contract research organizations and other key stakeholders, improved access, diversity and experience are clear attributes of modern virtual trial offerings. In most cases virtual trials that include virtual patient matching, consents, education and onboarding can increase patient overall recruitment by between 50-65%. More advanced capabilities will enable early patient findings and create awareness of early intervention opportunities and even engage with patients via social channels.
When considering the average cost to develop and discover a medicine is between $2.6bn-6.7bn, Accenture estimates that with continued conservative adoption of virtual trials the industry can save between $630m-820m per asset. This is not just significant from a cost perspective, but mitigates the barriers of geography, hurdles of consent, and recruitment, to list a few of the advantages.
The future is here. With the tools available, and the willingness to deploy them, the industry could (1) accelerate to equitable representation in clinical research enabling them to (2) more rapidly recover from the pauses, halts and slowdowns of their pipeline due to the pandemic and (3) be more resilient in future major regional and world events. This could change the face of medicine and the clinical outcomes for some of our most impacted communities.
About The Authors
Sanskriti Thakur is the global life science research lead for Accenture and Nicole Paraggio is a managing director at Accenture, a global professional services company. Thank you for the contributions of Poorna Iyer, an industry research adviser for the life science sectors, and Emily Spiegel and Obi Nnebedum.