Off The [Cold] Chain: New Ideas For Managing Ultra-Cold COVID Vaccines
Executive Summary
Off the grid ultra-cold storage “Vaccine Pods” and mRNA vaccines reformulated to be stable at room temperature are two innovative ways to address the problem of getting the whole world vaccinated against COVID-19.
When Larry Davis recorded “Texas Flood” in 1958 – “Well it’s floodin’ down in Texas/all the telephone lines are down” – he probably never imagined that a blizzard would knock them out in 2021. And not just the telephone lines. During the historic snowstorm in February, the Texas Department of State Health Services announced that thousands of COVID-19 vaccines would not arrive as planned, due in part to concern about power outages and the potential for vaccine spoilage. Even if the vaccines did arrive on time, people might not be able to travel to vaccination sites.
It does not take a blizzard to interrupt the cold chain infrastructure needed to keep vaccines at the correct temperature, from manufacturing site to injection. And it is not clear how many COVID-19 vaccines have been wasted so far. In the US, the CDC requires vaccination sites to report the number of wasted doses, but some state governments are not complying, according to reporting from ProPublica. Many states, however, have reported thousands of wasted doses, due to problems maintaining ultra-cold refrigeration, broken vials or other shipping issues.
Globally, the World Health Organization estimated that 50% of vaccines are wasted each year, due to inadequate temperature control and shipping problems. In the world’s poorest countries, only 10% of health care facilities have a reliable electricity supply, according to GAVI, the Vaccine Alliance. GAVI’s Cold Chain Equipment Optimisation Platform, established in 2016, is working to upgrade cold chain infrastructure in the 57 countries eligible for vaccine support, but many challenges remain.
Vaccine Pods
Edward Collins, a retired firefighter and battalion chief, a former CEO of a national retail mattress chain, a real estate agent and a bar owner based in Kansas City, Missouri, wanted to help when COVID-19 struck. In an interview with In Vivo, Collins said he had lost three friends in the fire department to COVID-19, and the virus was a contributing factor in his wife’s death last year. “At the beginning they said we’d need ventilators. I went to Home Depot [a home improvement retailer] and Micro Center [a computer and electronics retailer] and made a homemade ventilator for $88,” said Collins. “I published those plans for free on a website for COVID-related products … but then there wasn’t the rush on ventilators like everyone thought, but I just wanted to help.”
 Edward Collins, founder and CEO, Vaccine Pods
Edward Collins, founder and CEO, Vaccine Pods
Next, Collins started looking at the supply chain for products requiring ultra-cold storage. “If you look from the manufacturer to the end user, it’s fraught with all kinds of problems,” said Collins. “We’re talking about saving billions of lives, and we’re entrusting that to cardboard boxes with dry ice … you can recognize the breaks almost immediately.” He considered producing dry ice, or opening up freezer farms in existing facilities to assist the supply chain, but ultimately decided to launch a new company from stealth – Vaccine Pods – on 27 January, 2021. Through a partnership with HCI Energy LLC, a power solutions company, and Stirling UltraCold, an ultra-low temperature freezer company, Vaccine Pods are shipping containers capable of storing vaccines safely with or without an external power supply (see Exhibit 1).
| Size | Storage Capacity* | Power Sources | Days Off Grid |
| 46 lbs. portable freezer | 7,000 doses | Battery, plug-in | 3-4 days (1.5 hour recharge time) |
| 10' pod | 30,000 doses | Batteries, generator port, plug-in | 6 days without external power source |
| 20' pod | 60,000 doses | Batteries, solar array, wind turbine, generator with fuel tank (propane or diesel), plug-in | Indefinite length of time without external power source |
Source: Vaccine Pods
*Vaccine dose capacity numbers vary slightly according to product type
The partnership with HCI Energy, also based in the Kansas City area, came about serendipitously: Collin’s patent attorney also had HCI Energy as a client, and connected Collins with HCI Energy CEO Ray Ansari. “It made sense to bring in [HCI Energy] as a strategic partner with us to provide their technology base, and pair it out to Sterling UltraCold’s freezers, and have the whole package put together in the Vaccine Pods,” said Collins. During the discovery phase of putting the company together, Collins realized that Stirling UltraCold “was the only commercial grade lab freezer in the world capable of reaching the temperatures we needed, but also adjustable to a broad range of temperatures to accommodate every vaccine coming out.” Stirling UltraCold sold Collins a demo unit from one of its showrooms, and he brought it to Kansas City. There, Collins combined the technology from HCI Energy and Stirling UltraCold together, inside the pod.
The Vaccine Pods are available in 10-foot and 20-foot models, with an additional “rapid intervention freezer,” a Stirling UltraCold portable freezer ULT-25 model. All three models are equipped with telemetry systems for monitoring freezer health, temperature, ambient temperature inside the 10’ and 20’ pods, solar wattage produced, power levels inside the pod, and fuel levels inside of the back-up generator, said Collins. “We have 24-hour real-time monitoring for all of this, which allows us to dispatch fuel trucks against a client’s fuel count if needed, to maintain power at all times.”
Vaccine Pods can be transported on “planes, trains and automobiles,” said Collins, including roll-off tow trucks, gooseneck trailers and flatbeds, and they are also rated for cargo ships and can be stacked, “even with the solar array tucked away on the inside.” The pods are capable of withstanding 145 mile-an-hour winds, and are locked down on site, once in place. The 20’ pods having air conditioning and heating systems and can turn a parking lot into a vaccine administration site. “People can drive up to them, come in, we can install a table inside [the pod] and give vaccinations right there,” said Collins. Security keys and codes are used for access, which can also be granted remotely. Vaccine Pod security is “not quite to the level of a Brink’s Truck, but it’s pretty close.”
Collins believes his initial customers will be government agencies and NGOs. “We’ve had great conversations in Washington DC, with congressmen and congresswomen, and they hand-walked our units over to multiple agencies focused on vaccine deployment and vaccination plans,” said Collins. “We’ve had incredible outreach from local governments, health departments, bureaus of federal prisons,” as well as NGOs such as USAID, UNICEF and the Gates Foundation. “Hopefully, as we get through communication and discussions with people in DC, we can lump everybody under the Defense Production Act to assure that we have the supply chain we need to fill everybody’s order.”
Pricing for the Vaccine Pods ranges from $38,000 to $40,000 for the portable rapid intervention freezer, which holds 7,000 doses and can go off-grid for three to four days before recharging. The 20’ pod, which can hold two 30,000 vaccine capacity Stirling freezers, for a total of 60,000 vaccines, with “all the bells and whistles, including redundant power supply, and depending on how many batteries you include, comes in at about $189,000,” said Collins, estimating that 30,000 doses might be worth around $6m, making extra power redundancies a sound insurance policy.
After COVID, Collins hopes to expand into different areas. With the launch of mRNA vaccines, “we are going to have ultra-low temperature vaccines for a long time, and they will expand into different areas,” said Collins. “But we’re also looking at any [drug] that requires any type of refrigeration or freezing. Whether it’s an mRNA vaccine or more traditional vaccines, we’ll be able to go forward and help with other inoculations like rheumatic fever and polio in remote areas of the world.”
Thin Film Freezing
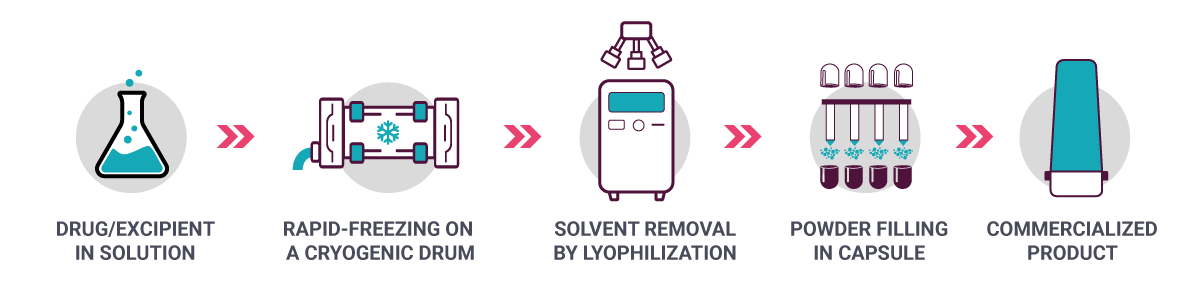
What if vaccines – and monoclonal antibodies, for that matter – no longer needed to be refrigerated, and remained stable at room temperature? Robert O. Williams III, inventor of thin film freezing technology (TFF) and scientific consultant to TFF Pharmaceuticals, is hoping to accomplish just that. In an interview with In Vivo, Williams, who is also head of the College of Pharmacy’s Molecular Pharmaceutics and Drug Delivery Division at the University of Texas at Austin, said TFF technology began as a research collaboration 20 years ago with the Dow Chemical Company. “We were interested in an alternative delivery route for drugs, including biologics, so that we could get them to the lungs,” said Williams. TFF technology is created through a process involving rapid freezing in a cryogenic drum and lyophilization (see Exhibit 2).
Today, TFF Pharmaceuticals, based in Austin, TX, is carrying that project forward. “We started working on technology about five years ago specifically for biologics like the mRNA-based vaccines,” said Williams. “It’s fortunate that we did, because now we’ve built up a significant amount of know-how and intellectual property and publications on applying thin film freezing as an alternative for stability storage, which doesn’t require cold chain.” Even before the advent of mRNA vaccines, refrigeration has always limited biologic distribution, particularly in countries without advanced cold chain storage capabilities,” said Williams. “COVID-19 vaccines have brought the seriousness of this issue front and center.”
While TFF Pharmaceuticals has not tested its technology on the Pfizer/BioNTech and Moderna mRNA vaccines specifically, the company has tested it on “several different mRNA [products] contained in lipid nanoparticles … We are working with those now and have had successful results with applying thin film freezing to stabilize mRNA lipid nanoparticle-based therapeutics,” said Williams. “They’re similar to the [BioNTech and Moderna] vaccines, and directly applicable.” TFF Pharmaceuticals has not disclosed which mRNA products it is testing with TFF technology but has described its partners as “the top pharma companies in the world,” said Williams.
TFF Pharmaceuticals has also developed an inhaled formulation of Veklury (remdesivir), Gilead’s antiviral therapy, which received FDA approval on 22 October, 2020 for patients with COVID-19 requiring hospitalization. The approval followed an Emergency Use Authorization last May. Williams developed the inhaled remdesivir formulation in his lab, sponsored by TFF Pharmaceuticals, and said it “works really well … we just completed a study in hamsters, the preferred COVID infection animal model, and have recently published a paper [on those results].”
Williams believes that an inhaled formulation of remdesivir offers “a lot of benefits over the intravenous form currently approved, and what we believe is a nebulized form of remdesivir that Gilead has stated publicly that they are developing and testing.” The question now for TFF Pharmaceuticals is whether and how to move the program forward, given that Gilead has a patent on the raw material. “We’d like to partner with them, so that’s where it stands,” said Williams. TFF Pharmaceuticals also announced a joint development and collaboration agreement with Augmenta Bioworks on 2 November, 2020, to reformulate select Augmenta monoclonal antibodies into inhaled treatments for COVID-19.
 Robert O. Williams, inventor of thin film freezing
Robert O. Williams, inventor of thin film freezing
In press materials, TFF Pharmaceuticals announced that it has identified 18 potential drug candidates that could be transferred to inhaled or oral formulations. The company has a partnership with Denmark-based UNION Therapeutics to develop an inhaled formulation, and an “improved” oral formulation of niclosamide, an antihelminthic drug used to treat parasitic infections, and with potential applications in a range of other diseases. Last October, the company announced “positive preclinical immunogenicity and efficacy data” from TFF formulated “universal influenza hemagglutinin recombinant vaccines,” through a partnership with the University of Georgia’s Center for Vaccines and Immunology.
Williams said TFF Pharmaceuticals aims for “room temperature storage for two to three years for a typical kind of pharma product.” With biologics, “when you have a dry powder that has the properties of our TFF process powder, we have examples of vaccines and examples of different proteins where we are able to go out for about a year and half or two years now, with stability that we’re following. Every molecule is unique, but that’s our goal, and we think it’s achievable.”
The benefits of TFF formulation could go beyond room temperature storage, explained Williams. “For each type of active material, whether it’s niclosamide, or a monoclonal antibody, or the influenza vaccine, they are nuanced amongst themselves.” But the key benefits may come in the form of lower dosages, and a reduction in off-target side effects. That had been demonstrated in hamster models with respect to TFF formulated niclosamide, said Williams.
For many in the biopharmaceutical industry, inhaled biologics will immediately call to mind the twin failures of Pfizer and Mannkind’s inhaled insulin products, which ultimately spooked physicians and patients over pulmonary risks. Williams acknowledged that risks have to be determined case by case during the clinical process, but said “a lot has been learned about applying the different larger molecules like proteins and peptides into the lungs” since the excitement around inhaled insulin. “With TFF Pharma, we’ve talked to 30 or 40 companies now, and the scientists – I’m generalizing – but the scientists that I talked to are not concerned about [pulmonary risks] now. They think there's always a risk, but it's one worth taking to apply these biologics into the lung. Their limiting factor has been that they didn't have a way to do it, because there was no way to form an inhalable powder with a biologic that preserves the biologic's chemical integrity, its activity. But we can do it with thin film freezing technology.”