Editing The Future
Executive Summary
Gene editing is positioning itself as the latest evolution of genetic therapies and is due for an inflection point in 2022 as a number of milestones will be reached.
Like any new technology that has been decades in the making, gene therapy is evolving. The classical approach for in vivo gene therapies involves the delivery of additional genetic information to particular cells in order to express a protein that will be curative in a given disease state. With the persistence of notable challenges such as vector safety issues, tissue targeting and durability, there is intense selection pressure on the technologies employed. Newer approaches to gene therapy are garnering a lot of attention, being a standout theme at the annual J.P. Morgan Healthcare Conference in January 2022. Ahead of a catalyst-rich year to come, this article serves as a primer for the various editing technologies and players. Should their potential be fulfilled, then gene editing will hasten the demise of earlier and inferior generations of cell and gene therapy platforms.
Gene Editing Technology Background
Since the first publications in the early 2010s, gene editing has gained rapid prominence and recognition for its utility across the life sciences. CRISPR pioneers Professors Charpentier and Doudna shared the 2020 Nobel Prize for Chemistry and have spawned a number of gene editing biotechs seeking to cure genetic disorders, while its therapeutic application extends more broadly to cancer and infectious disease, not to mention synthetic biology and crop science.
Three major differences set gene editing apart from the classical gene therapy approach. The first is that editing does not introduce new genetic material into cells, but rather modifies the nucleobases that already exist. This is achieved through creating breaks in one or both DNA strands and relying on repair mechanisms to restore the double helix. Secondly, as well as triggering the production of functional proteins, gene editing can also be used to silence the expression of disease-causing proteins, depending upon the cutting and repair mechanisms employed. Lastly, because the target DNA is edited directly, such changes are preserved through cell division; the payload of classic gene therapies persists in the nucleus and does not necessarily integrate into the genome.
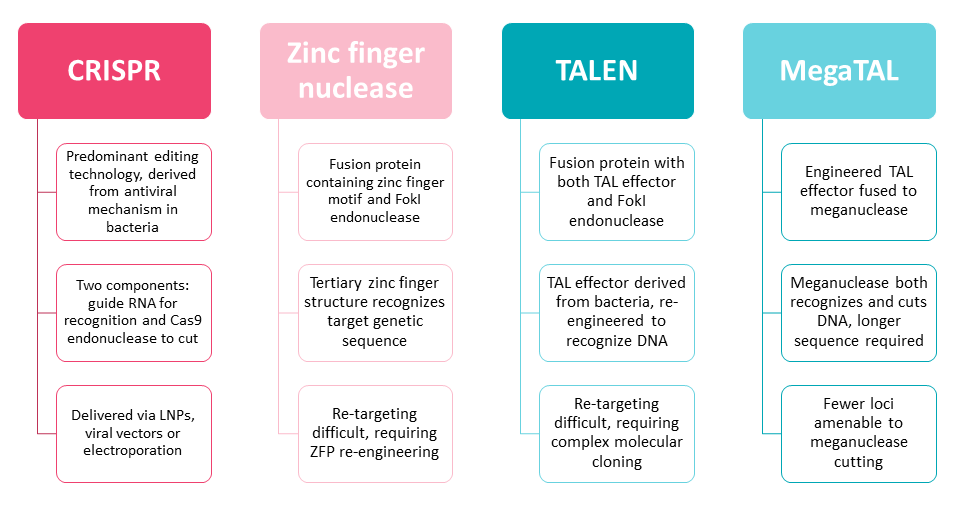
Gene editing itself is best described as a concept, as the science and mechanics of editing are diverging at pace along discrete lines, as shown in Exhibit 1. There are key differences in the use of different recognition domains that target various stretches of DNA and the choice of cutting enzyme, resulting in different types of DNA breaks.
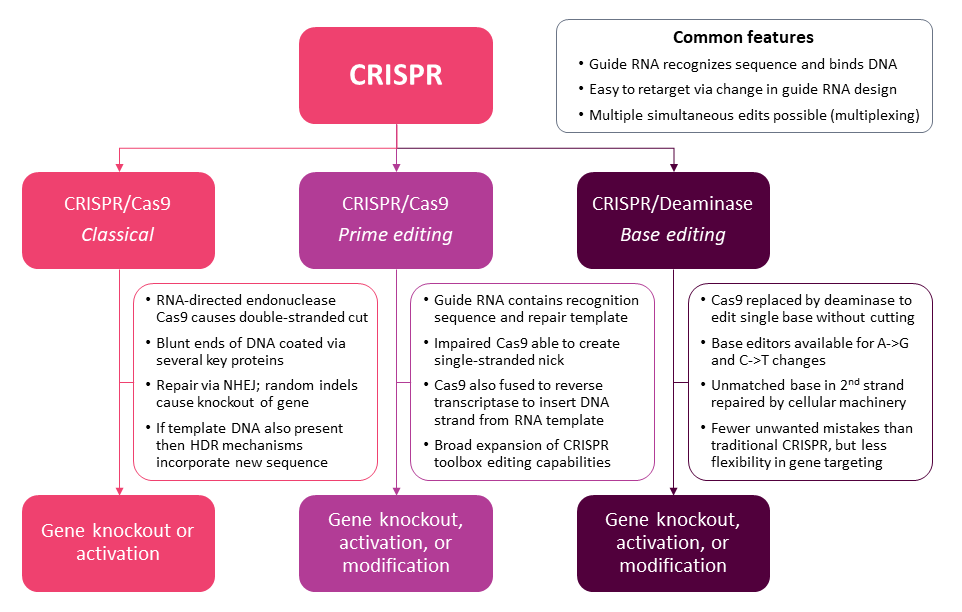
Exhibit 2 highlights recent advancements within CRISPR that allow for direct editing and substitution of bases with single stranded DNA breaks, while gene editing is also stretching to encompass the insertion of new genetic information. As with gene therapy, editing can also take place in vivo or ex vivo, the latter being used as a tool to reprogram cell therapies with modified genetic information such as CAR-T cells.
Exhibit 1. Gene Editing Classification And Technologies
Source: Informa Pharma Intelligence
Exhibit 2. Evolution And Expansion Of CRISPR Capabilities
Source: Informa Pharma Intelligence
CRISPR is emerging strongly as the preferred mechanism for gene editing. Being RNA-based, it is more flexible and scalable than other methods that require the identification and creation of complex proteins for each new application. Another tremendous advantage is its ability to simultaneously edit multiple loci, allowing for much greater efficiency. Next-generation iterations of the CRISPR platform hold the promise of an expanded toolbox. Through the use of single DNA breaks, or nicks, and specific template/enzyme pairings, the resulting genetic sequence can be modified with much greater precision and fidelity. Base editing allows the identification and editing of the genome at the level of a single base pair, while prime editing is designed to change longer stretches of DNA and replace with an exact sequence as specified in an RNA template without the risk of random indels (insertions or deletions).
Gene editing is still very much in its infancy. It has undoubtedly proven its worth many times over as a tool for biological research, but its true potential in the healthcare setting lies as a therapeutic. In order to be used as a drug, there are many hurdles that will need to be overcome. Many of these issues are shared with the current crop of gene therapies, such as assurances over long-term safety, optimizing delivery and expression in the right tissues, and building mechanisms by which these complex therapeutics can be reimbursed. Despite the scientific promise of gene editing, there are practical realities that mean patients and physicians may prefer the familiarity and reliability of conventional treatments. Still, 2022 is a very exciting time to observe gene editing, as the pipeline grows at remarkable pace and the first glimpses of clinical data emerge.
Remarkable Growth In The Gene Editing Drug Pipeline
Gene editing is the hottest area in biopharma drug development. Its growth since 2016 – doubling every two years – is unmatched by any other therapeutic modality, outstripping all other forms of cell, gene or RNA therapy. This momentum is carrying strongly into the turn of the year, with 279 active gene editing assets tracked by the biopharma pipeline database Pharmaprojects in January 2022 (see Exhibit 3).
The 279 active assets currently in development include both in vivo drugs, where the gene edit itself is the therapeutic, akin to conventional gene therapies, as well as ex vivo editing approaches applied to cell therapies (see Exhibit 4). Over three quarters of these programs are in vivo gene editing (217 out of 279). Although ex vivo editing is the minority, partly due to alternative options by which cells can be reprogrammed, this approach is more validated. Of the 62 ex vivo gene edited cell therapies, 21 are listed in various stages of clinical development. Just 12 in vivo gene editing programs have moved into the clinic. For in vivo editing, there are the additional challenges of delivery and specificity, whereas cells edited in a petri dish can be analyzed and purified before reinfusion.
As with any type of drug with a mechanism operating at the DNA level, the simplest clinical application for gene editing is to correct faults in monogenic diseases. These inherited genetic diseases are often rare and underserved by conventional treatments, leaving considerable unmet need. The same can be said for diseases in which cell therapies are gaining prominence. Accordingly, when the gene editing pipeline is stratified by therapeutic area, rare disease is a clear standout for both in vivo and ex vivo methods (see Exhibit 5). Looking beyond this broad descriptor of disease prevalence, the two distinct leading therapeutic areas for gene editing are neurology and oncology.
Neurology is led strongly by in vivo editing approaches, in spite of the challenges in reaching the central nervous system associated with common delivery methods such as lipid nanoparticles and viral vectors. Neurology also contains a range of different developmental diseases that broadly result in neurological symptoms, even if the origin is due to a missing or deficient enzyme in the liver. The therapy areas of such drugs are not mutually exclusive, and they can also be classified as metabolic and/or musculoskeletal in certain cases. In vivo gene editing is also commonly being investigated in rare inherited retinal disorders, although the potential use cases span almost every possible therapeutic area.
By contrast, ex vivo, gene edited cell therapies are largely concentrated in oncology. Gene editing can be harnessed to produce CAR-Ts more efficiently than other genetic techniques and unlocks the potential for allogeneic cell therapies with reduced risk of rejection. Although oncology is the most numerous setting, it is hematology where gene edited cell therapies are the most mature. Companies such as CRISPR Therapeutics and Intellia Therapeutics have clinical-stage programs targeting patients with beta-thalassemia and sickle cell disease using engineered hematopoietic stem cell therapies designed to produce fetal hemoglobin. There are also ex vivo gene editing applications in patients with immunodeficiency and to manage transplant rejection.
Key Players Jockeying For Position At J.P. Morgan 2022
Gene editing was undoubtedly one of the major themes of the 2022 J.P. Morgan Healthcare Conference, held virtually in lieu of its long-running residence at the Westin St. Francis hotel in San Francisco, CA. Absent any announcements of acquisitions, it was alliances that came under the spotlight. The gene editing biotechs with deals to announce – Beam Therapeutics and Mammoth Biosciences – received external validation of the potential of their platforms. On the large pharma side of the deal, it was also revealing to see the first movers seeking to adopt this new pipeline technology. Pfizer placed one of the largest upfront bets thus far ($300m) in its alliance with Beam, which ranks behind only the broad partnership between Vertex and CRISPR Therapeutics signed in 2015 and updated in 2021. (Also see "Deal Watch: Bristol, Pfizer Lead Off J.P. Morgan Week With Two Deals Apiece" - Scrip, 11 Jan, 2022.)
Regardless of whether there were deals to be announced or not, all of the major gene editing players gave presentations to investors and potential partners on the status of their technology and pipeline. Table 1 contains a non-exhaustive list of the major players, ranked by post-J.P. Morgan market capitalization, and their various updates are summarized in the following section. Companies using gene editing technology but primarily considered as cell therapy companies and without proprietary editing capabilities are not included.
Intellia Therapeutics
Currently possessing the highest valuation of any gene editing biotech, in part due to being the first to successfully deliver in vivo CRISPR gene editing to human patients, Intellia has a broad technology platform and pipeline. Its landmark drug NTL-2001 achieved dose-dependent knockout of TTR, the misfolded protein responsible for ATTR amyloidosis, with an 87% reduction at the higher dose exceeding that of more conventional RNA-type drugs. (Also see "Intellia Achieves Gene-Editing Breakthrough With First In Vivo CRISPR Therapy" - Scrip, 28 Jun, 2021.) This trial, which originally enrolled six patients, is now being expanded to up to 74 patients with a mix of polyneuropathy and cardiomyopathy. These results will inform the optimal dose to progress into pivotal clinical development, although there are no available timelines for this yet. Intellia’s second in vivo candidate NTL-2002 is also in a first-in-human study for hereditary angioedema. The company is aiming to share interim data in the second half of 2022, likely containing a similar look at early dose response and gene silencing as per the NTL-2001 publication in the New England Journal of Medicine.
Intellia’s approach to ex vivo edited cell therapies is centered on T cell receptor (TCR) therapies, rather than the more common CAR-T approach. Gene edited TCRs offer the promise of an off-the-shelf approach that can target a broad range of tumor-specific antigens, not limited to those expressed on the cell surface. The potential of TCRs extends not only to hematological malignancies, but also solid tumors where the antigen is intracellular and/or constrained by the tumor microenvironment. This means that Intellia will not be competing in the increasingly crowded anti-CD19 space, but rather targeting its cell therapies against new unmet need. Its lead program NTL-5001 is due to begin Phase I/II development in patients with AML during 2022. As with some of its competitors, Intellia has also created an ex vivo hematopoietic stem cell therapy for patients with sickle cell disease. The drug is of scientific importance for Intellia’s editing capabilities, although its lateness to the clinic diminishes any strategic priority. A 30-patient Phase I/II sponsored by its partner Novartis is open for enrollment, although Intellia is not providing timelines.
CRISPR Therapeutics
While not actually presenting at J.P. Morgan, CRISPR Therapeutics is nevertheless a founding gene editing company with a number of important milestones in 2022. On the ex vivo side, CRISPR’s lead program CTX001 is partnered to Vertex for development in beta-thalassemia and sickle cell disease. Preliminary Phase III data are available for up to 24 months, showing clinically meaningful levels of fetal hemoglobin, which translates into freedom from symptoms and a potential long-term cure. Regulatory filing is expected before the end of the year as the dataset matures, setting up CTX001 to become the first approved CRISPR/Cas9 drug. It would also be first-to-market in sickle cell disease and in likely competition with bluebird bio’s classic gene therapy beti-cel for beta-thalassemia. The head-to-head contrast will be a revealing assessment of CRISPR’s precision compared to lentiviral insertion for hematopoietic stem cell therapies.
CRISPR is also set to achieve milestones for its wholly owned allogeneic CAR-T cell portfolios, including CTX110 (anti-CD19), CTX120 (anti-BCMA), and CTX130 (anti-CD70). Phase I data for its CARBON trial of CTX110 in B cell malignancies have already been released, with efficacy not far behind the benchmark set by autologous CAR-Ts, albeit with a patient death at the highest dose. (Also see "CRISPR Therapeutics Moves Into Off-The-Shelf CAR-T Lead, But Durability Test Awaits" - Scrip, 13 Oct, 2021.) This provides the rationale for CRISPR to expand the CARBON trial in Q1 to provide a pathway to registrational filing. First-in-human data for CTX120 and CTX130 will be available during the first half of 2022. These may become the first gene edited CAR-Ts to show efficacy with non-CD19 constructs, raising confidence in CRISPR’s wider platform and the broader potential of allogeneic cell therapies.
Beam Therapeutics
Beam is a pioneer in base editing, a CRISPR-based technology that incorporates the precise substitution of single bases without the creation of random indels. Although the technology is constrained in terms of the flexibility of possible edits, there are nevertheless an extraordinary number of single point mutations that could be targeted for therapeutic benefit. Despite its lofty valuation, Beam remains a preclinical-stage biotech, albeit with a number of partnerships relating to its editing and delivery technologies. Tellingly, Pfizer CEO Albert Bourla revealed that his company had evaluated all of the editing players before settling on its partnership with Beam, revealing “base technology has the most promise.” The four-year collaboration allies Pfizer’s mRNA-LNP experience through COVID-19 with Beam’s editing and LNP technology.
Beam expects to progress its lead asset into clinical trials in the first half of 2022. BEAM-101 is an autologous hematopoietic stem cell therapy containing an edit that activates production of fetal hemoglobin. Preclinical data suggest that base editing achieves superior editing compared to classical CRISPR cell therapies with this mechanism, alongside the theoretical benefits associated with greater accuracy. The Phase I/II BEACON-101 trial will present the first opportunity to directly compare base editing to CRISPR Therapeutics’s allogeneic therapy CTX001 in patients.
Beyond this, Beam anticipates filing INDs for BEAM-102 (sickle cell disease; correction of adult hemoglobin) and BEAM-201 (CAR-T for T cell leukemia) by the end of 2022. These are both ex vivo therapies, although Beam has earlier research projects seeking to capitalize on the potential for in vivo base editing via LNP delivery. Although these remain at least a year away from the clinic, initial programs will target liver indications such as GSD1a and A1AD. Beam will now separately begin working with Pfizer on in vivo editing for discrete targets in the liver, muscle, and central nervous system.
Editas Medicine
Editas is seeking to differentiate itself from other gene editing players both through its gene editing technology and therapeutic area strategy. The company has proprietary RNA chemistry and modified nucleases such as Cas12a, which is designed to produce higher editing productivity. While it is following CRISPR, Intellia and others in establishing clinical proof-of-concept for its CRISPR/Cas9 capabilities in beta-thalassemia and sickle cell disease (EDIT-201), Editas also has first-in-class programs in ophthalmology and ex vivo editing of induced pluripotent stem cells (iPSCs).
The company achieved a major milestone in 2021 with the first human data for EDIT-101, an in vivo AAV-delivered CRISPR/Cas9 therapy for patients with Leber congenital amaurosis 10 (LCA10). The BRILLIANCE trial showed anecdotal efficacy, leading to a mixed reaction from investors. SC145159 Through 2022, Editas will be reporting additional data for higher doses, as well as completing dose escalation in pediatric cohorts. It will be important that EDIT-101 produces more tangible gains in visual acuity after a longer follow-up period for Editas to move forward with confidence into a registrational trial.
The company’s lead drug from its gene edited iPSC platform is EDIT-202, a natural killer cell therapy in development for solid tumors. It contains two gene knockouts to boost the immune response and penetrate tumor microenvironment, in addition to two knockins for proliferation and persistence. Editas will begin IND-enabling studies for EDIT-202 this year. Also, on the cell therapy side, Editas has a collaboration with Bristol Myers Squibb for alpha beta T cells with multiplex editing, with the first candidate now in IND-enabling studies.
Verve Therapeutics
Supported from technology licensed from Beam Therapeutics, Verve is applying base editing to genetic cardiovascular diseases. Following fundraising activities exceeding $400m in 2021 and the completion of IND-enabling non-human primate studies, Verve is looking towards a number of milestones in 2022. The most important of these will be IND filing for VERVE-101 and the treatment of the first patient with heterozygous familial hypercholesterolemia (HeFH) towards the end of the year.
VERVE-101 is an in vivo base editor delivered via LNPs that turns off the PCSK9 gene via a single A->G change. PCSK9 inhibition is already well validated for dyslipidemia through monoclonal antibodies and RNA interference, but Verve’s approach is designed to achieve a permanent therapeutic effect in individuals with the specific LDLR mutation that causes HeFH. The company’s planned Phase I trial will recruit approximately 40 HeFH patients across three dose arms, evaluating safety and reduction in PCSK9, LDL-C and ApoB. Should VERVE-101 become established in HeFH, subsequent lifecycle management may see the drug eventually tested for broader patient populations, such as those with confirmed atherosclerotic cardiovascular disease, and even as the first primary care gene editing therapy for cardiovascular risk reduction.
Verve is also planning to select a lead candidate from its ANGPTL3 program and initiate IND-enabling studies in the second half of 2022. This is expected to result in a clinical program for homozygous familial hypercholesterolemia (HoFH), and the ANGPTL3 base editor may eventually be amenable to sequential dosing with VERVE-101 in broader populations. While Verve is clearly some way behind its gene editing competitors in terms of clinical timelines, its strict portfolio focus on in vivo editing of cardiovascular disease is unique.
Will Editing Eventually Replace Classical Gene Transfer?
Gene editing remains very much in the shadow of classical gene therapy, which involves the transfer of new genetic material rather than overwriting or modification of existing sequences. Gene therapy has a vastly superior amount of clinical data supporting its use in both the in vivo and ex vivo settings. Nevertheless, it is tempting to be drawn in by the promise of gene editing techniques, which have tremendous platform potential. The ability to make multiple genetic modifications at a time, coupled with the precision by which these changes can be made, suggest that gene editing can be scalable in a way that earlier generations of gene therapy are not. Furthermore, classical gene transfer approaches are yet to become mainstream, constrained by the challenges of vector safety, durability, tissue targeting, and ultimately market access.
As momentum behind traditional gene therapies stalls, recent scientific advances in the editing field – in particular CRISPR – are alluring. The unbridled optimist may go so far as to suggest that gene editing will eventually make gene transfer entirely redundant in the clinical setting. 2022 will be an important, albeit small, step in this direction, as clinical datasets for the first generation of editors mature and proof-of-concept is established in new therapeutic areas. However, it is practical reminder that advances in drug platforms are typically measured over decades and not individual years. CRISPR remains very nascent and has yet to face the inevitable clinical setbacks that characterize emerging science. It will be only be through the trials and tribulations that the true potential of gene editing will ultimately be recognized.
For more on this topic see our latest report on gene, cell and RNA therapies with The American Society of Gene and Cell Therapy (ASGCT).