News In Brief
This article was originally published in The Gray Sheet
Executive Summary
J&J scrutiny continues. New product launches. More news.
J&J scrutiny continues
Federal and state authorities are investigating Johnson & Johnson’s marketing practices for its ASR XL hip device and its surgical mesh products, the company disclosed in its annual report filing to the Securities and Exchange Commission Feb. 22.
Since August, J&J’s DePuy Orthopaedics Inc. unit has been targeted with several requests for information from the United States Attorney's Office for the District of Massachusetts and the Civil Division of the United States Department of Justice related to the 2010-recalled ASR hip, requests with which the firm is cooperating. The government is investigating whether any false claims or false statements were submitted affecting federal health care programs in connection with the marketing and use of the ASR device, J&J said. The federal inquiry comes as J&J faces more than 10,000 product liability lawsuits related to ASR. (See (Also see "News In Brief" - Medtech Insight, 28 Jan, 2013.).)
J&J also disclosed that in October 2012, the company was contacted by the California Attorney General's office regarding a multistate attorney general investigation of the marketing of surgical mesh products for hernia and urogynecological purposes. The company last year initiated a phased market withdrawal of several of its vaginal mesh products, which have also triggered a wave of patient lawsuits and are under safety scrutiny as a product class. (See (Also see "J&J/Ethicon Begins Worldwide Withdrawal Of Vaginal Mesh Products" - Medtech Insight, 11 Jun, 2012.).)
DePuy Synthes shoulder
DePuy Synthes Joint Reconstruction announced the worldwide launch of its 510(k)-cleared Global Unite platform shoulder arthroplasty system Feb. 19. Global Unite is a modular system that allows 72 different sizing configurations. And, according to the Johnson & Johnson company, it is the only modular system to provide a suture collar, which allows secure anatomic reconstruction and helps return the shoulder to a natural anatomical position. The system is indicated for press-fit or cemented fixation.
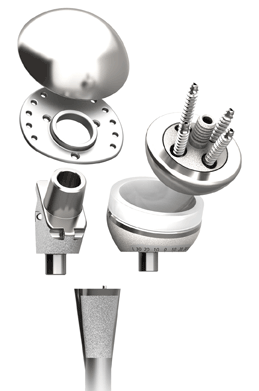
Global Unite platform shoulder arthroplasty system
Photo: Johnson & Johnson/DePuy Synthes Joint Reconstruction
New breast implant approval
FDA approved Allergan Inc.’s latest silicone breast implant Feb. 20. The firm says the Natrelle 410Highly Cohesive Anatomically Shaped Silicone-Gel Filled Breast Implant is designed to mimic the slope of the breast better than traditional round implants. In addition, the silicone gel in the device contains more cross-linking compared to Allergan’s 2006-approved Natrelle implant, to help the implant hold its shape over time while remaining soft to the touch. The new implant was approved based on seven years of data from 941 women, despite continued safety scrutiny of the breast implant product class. FDA says most complications and outcomes associated with the product reflect those found in previous breast implant studies. The agency cautions that the implants should not be considered “lifetime devices” and that long-term monitoring is essential. Allergan is required to conduct multiple post-approval studies for the Natrelle 410 implant.
Emergency-use device tax bill
Rep. Michael Turner, R-Ohio, has introduced House H.R. 581, a bill to exempt emergency use medical devices from the 2.3 percent excise tax. The “First Responder Medical Device Tax Relief Act” would cover devices “of a type furnished by first responders or ambulance services in providing out-of-hospital or pre-hospital care, or transport to a medical care facility,” according to the legislation. H.R. 581 is being co-sponsored by four Republicans in the House. No similar bill has been proposed in the Senate.
Unnecessary tests
A total of 25 medical specialty societies have identified more than 130 tests, procedures or medication therapies that are commonly ordered but not always necessary in the Choosing Wisely campaign. Lists of the questionable tests and procedures, such as use of percutaneous feeding tubes in patients with advanced dementia and the automatic use of CT scans to evaluate children’s minor head injuries, were released Feb. 21 by the ABIM Foundation during a press conference in Washington, D.C. The campaign aims to promote conversation between physicians and patients by helping patients choose care that is supported by evidence, not duplicative of other tests or procedures already received, free from harm and truly necessary. Each specialty society participating in the campaign identified five specific tests or procedures that are commonly done in their profession. Seventeen specialty societies participated in the campaign this year in addition to the original nine organizations that each released lists in April 2012. The ABIM Foundation also announced that it received a $2.5 million, 28-month grant from the Robert Wood Johnson Foundation to advance the campaign.
Terumo to target U.S. urology specialty
Terumo Interventional Systems, a business unit ofTerumo Medical Corp., announced Feb. 19 the launch of a new group focused on direct sales, marketing, support and distribution of products specific to the urological specialty beginning April 1. “The move is part of the company’s commitment to strengthening a direct relationship with urologists nationwide that currently use its market-leading Glidewire hydrophilic guidewire family of products,” the company said in a press release. This new strategy marks the end of a distribution agreement of Glidewire products to U.S. urology customers between Terumo Interventional Systems and Boston Scientific Corp. This is the second time Terumo is creating its own U.S. sales force to sell its guidewires and sheaths, following the end of a distribution arrangement with Boston Scientific. (See (Also see "Terumo To End Guidewire And Sheath Distribution Deal With Boston Scientific" - Medtech Insight, 24 Oct, 2005.).)
Boston Sci taps neuromod distributor in Japan
Fukuda Denshi Co. Ltd. will market Boston Scientific Corp.’s Spinal Cord Precision Plus neuromodulation pain-management system and accessories in Japan beginning April 1 under a deal announced Feb. 20. Boston Scientific says Fukuda Denshi's sales resources, distribution network and service capabilities will allow it to reach many more accounts, customers and patients in Japan.
Boston Scientific says the Spinal Cord Precision Plus System is the only available device with 16 independent current sources to precisely target patient pain. It is also the only pain-management neuromodulator that can be charged and controlled wirelessly, the company says. Boston Scientific acquired the Precision line in 2004 as part of the $740 million deal for Advanced Bionics Corp.
NxStage goes nocturnal in Europe
NxStage Medical Inc. announced Feb. 19 that it received a CE mark for nocturnal home hemodialysis with the firm’s System One, which will allow a patient to undergo dialysis while sleeping, usually between three to five nights a week. This “allows much greater time on dialysis without additional inconvenience to the patient or their family,” the Lawrence, Mass.-based company explained in a press release. The company expects to begin marketing System One for nocturnal dialysis in countries that recognize the CE mark in the second half of 2013. System One is the first FDA-approved hemodialysis machine that allows for more frequent dialysis in the home, although it is not specifically cleared in the U.S. for nocturnal home hemodialysis. (See (Also see "Home Dialysis Equipment Vendors Seek More Medicare Pay For Patient Training" - Medtech Insight, 27 Aug, 2012.).) NxStage is currently conducting a trial to support this indication.
In addition, NxStage Medical also announced Feb. 21 a CE mark for new high flow capabilities with System One. This capability will “enable dialysis flow rates which allow nephrologists expanded possibilities to adjust the duration and frequency of patient prescriptions with greater flexibility than they have today,” the company said.