Biopharma Dealmaking Quarterly Statistics, Q3 2013
A look at the financing, M&A, and alliance activity July-September 2013
Executive Summary
Biopharma financing in Q3 2013 totaled $4.6 billion, a 58% drop from the second quarter; Perrigo’s $8.3 billion acquisition of Elan made up more than half of the M&A dollar value; and marketed products made up the majority of licensed products.
Our review of biopharma dealmaking – for the third quarter of 2013. Our data come from Elsevier’s Strategic Transactions.
Financings
Biopharma companies closed the third quarter of 2013 with an aggregate $4.6 billion in financing, a 58% drop from the second quarter’s $11 billion total. Even excluding Valeant Pharmaceuticals International Inc.’s outlier $3.2 billion debt [See Deal] and $2.3 billion equity [See Deal] offerings in Q2, the third quarter still saw a 16% decline. (See Exhibit 1.)
Exhibit 1
Q3 2013 Biopharma Financing
By Deal Type ($M)
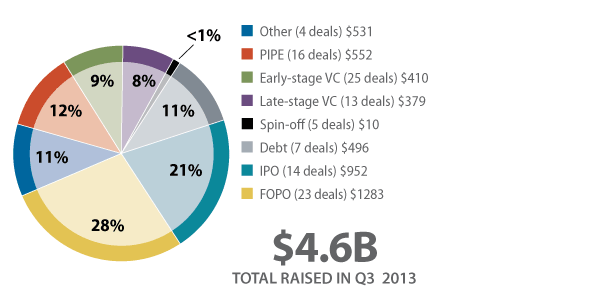
NOTE: Merrimack and KV Pharma’s concurrent debt and equity financings are accounted for separately in the Debt and FOPO, and Debt and PIPE categories, respectively.
Elsevier’s Strategic Transactions
The largest transaction was a royalty sale, a less common but often quite lucrative financing vehicle. Following its failed attempt to take over Elan Corp. PLC earlier in the year [See Deal], Royalty Pharma, whose business is to buy up revenue-producing IP (see (Also see "Royalty Pharma Earns Its Profits The Slow Way" - Pink Sheet, 9 Aug, 2013.)), paid Quest Diagnostics Inc. $485 million in cash for royalty rights to ibrutinib, a Phase III candidate for leukemia and lymphoma [See Deal], making up the majority of the Other category. (See (Also see "Quest Diagnostics’ Retrenchment Gives Royalty Pharma A Stake In Ibrutinib" - Pink Sheet, 18 Jul, 2013.).) Quest had been entitled to a single-digit royalty on the drug via its division Celera Corp., which sold ibrutinib plus other small molecules to Pharmacyclics Inc. in 2006. [See Deal] (Five years later, Pharmacyclics partnered the candidate with Johnson & Johnson’s Janssen Biotech Inc. (a division of Janssen Pharmaceutical Cos.) worldwide [See Deal].) The royalty sale leaves Quest to focus on its main diagnostic information services operations, and Royalty Pharma to reap the potential revenues from ibrutinib; in fact, less than a month after signing the deal with Quest, Royalty received $97 million by selling Aisling Capital and Clarus Ventures a 20% (10% each) cut of the ibrutinib royalties. (See (Also see "Aisling, Clarus Make Huge Bets On Royalties" - Scrip, 19 Aug, 2013.).) Despite the large figure that Quest procured for ibrutinib, this sale still only ranks fifth among the top-grossing royalty transactions to date in the biopharma industry. (See Exhibit 2.)
Exhibit 2
Top-Grossing Royalty Sales Of Biopharma Assets
(Since 1991)
|
Date |
Seller |
Buyer |
Subject Of Royalty |
Deal Value ($M) |
|
May 2012 |
Former owners of Fumapharm |
Royalty Partners |
Future earn-outs ($315M) that Fumapharm is entitled to under Biogen Idec’s 2006 $220M takeover of Fumapharm |
761 |
|
October 2006 |
Cambridge Antibody Technologies (a division of AstraZeneca) |
Royalty Pharma |
2.69% of Abbott Laboratories' rheumatoid arthritis drug Humira (adalimumab) |
700 |
|
June 2011 |
Prosidion (a division of Astellas Pharma) |
Royalty Pharma |
Patent estate and associated royalty stream and MS associated with dipeptidyl peptidase IV (DPP-IV) inhibitors for Type II diabetes |
609 |
|
July 2005 |
Emory University |
Gilead Sciences and Royalty Pharma |
Worldwide net sales of Emtriva (emtricitabine) for HIV |
540 |
|
July 2013 |
Quest Diagnostics |
Royalty Pharma |
Single-digit percentage of Phase III ibrutinib for leukemia and lymphoma |
485 |
Elsevier’s Strategic Transactions
During Q3, women’s health therapeutics firm Lumara Health Inc. started taking the first steps toward financial stability, emerging from the Chapter 11 bankruptcy it filed for in August 2012. (See (Also see "KV Emerging From Bankruptcy As Makena Sales Pick Up Steam" - Pink Sheet, 5 Sep, 2013.).) KV had been struggling because the FDA wasn’t enforcing the orphan drug exclusivity granted to the company’s key product Makena (hydroxyprogesterone), and subsequently KV was having problems marketing the pre-term labor drug. Plus, some state Medicaid agencies imposed reimbursement restrictions. In addition, KV was unable to renegotiate milestone payments to Hologic Inc., which out-licensed Makena to KV in 2008 [See Deal]. In an interesting turn, while KV was in Chapter 11 proceedings Makena sales actually grew. Further, under a reorganization plan announced in September 2013 – and accepted by the US Bankruptcy Court of the Southern District of New York – the company raised $375 million through a $100 million credit facility and a $275 million rights offering led by Capital Ventures International, Greywolf Capital, Kingdon Capital, Silver Point Finance, and Deutsche Bank [See Deal]. KV emerged from that arrangement with reduced debt: per terms of the plan, all of its existing senior secured notes will be paid in full in cash. With what some would consider a fresh start, KV is now positioned to tackle coverage and reimbursement issues for Makena.
Interested in getting an alert each time we publish a quarterly review of biopharma dealmaking? Click here![]() .
.
Follow-on public offerings remained strong, coming in at $1.3 billion and accounting for 28% of the money raised, the most of any financing type in the third quarter. Three of those transactions blew past $100 million, led by a $170 million secondary offering from orphan disease-focused Synageva BioPharma Corp. [See Deal] Early next year, the biotech hopes to move its SBC103 candidate for the rare disorder mucopolysaccharidosis IIIB into human testing. Since reverse merging with Trimeris in 2011 [See Deal], Synageva has completed four follow-ons (including the one in Q3), together netting $462 million. [See Deal] [See Deal] [See Deal] The other $100-million-dollar-plus FOPOs from the third quarter were achieved by critical care firm The Medicines Co. ($165 million [See Deal]) and cardiovascular-focused Amarin Corp. PLC ($122 million.) [See Deal]
With the IPO window now seemingly open, the influx of public debuts has increased significantly since the first quarter of this year. In fact, in Q3 nearly a quarter (21%) of the financings were IPOs, the largest percentage of total fundraising seen by the group in a single quarter since Shanghai Pharmaceuticals Holding Co. Ltd.’s monster $2 billion initial offering on the Hong Kong Stock Exchange in May 2011 [See Deal] drove the category to 35% of Q2 2011’s fundraising aggregate. (See Exhibit 3.)
Exhibit 3
Strong IPO Market In Q3 2013
IPOs As Percentage Of Total Financing Over The Past Five Years, By Quarter
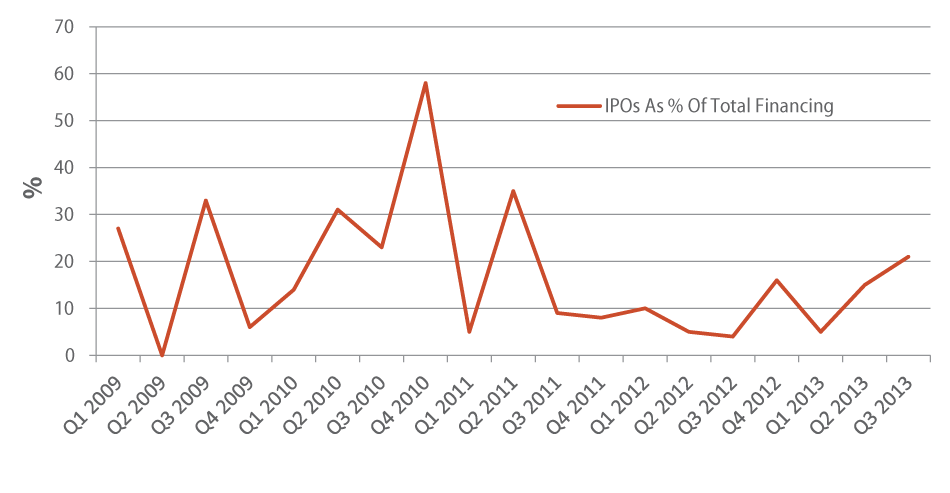
Elsevier’s Strategic Transactions
Together, 14 IPOs in Q3 raised $952 million, and five companies filed new S-1s. The majority of the biotechs that went public priced shares either at the midpoint, top end, or above their planned ranges, a contrast to the IPO haircuts to which we’ve become accustomed. Ophthotech Corp. netted the highest-valued IPO at $155.5 million, and at one point was trading at $33/share, $11 higher than its $22 IPO price. [See Deal] The six-year-old firm, formed by former OSI Eyetech executives, is testing Phase III Fovista (anti-PDGF agent) in combination with other anti-VEGF drugs for wet AMD. Ophthalmology must have caught the eyes of investors, as fellow eye care companies Aerie Pharmaceuticals Inc. [See Deal] and OphthaliX Inc. [See Deal] filed to go public as well in Q3 (note that Aerie completed its IPO in Q4 2013).
Early- and late-stage venture financing, together bringing in $790 million, was down 23% versus Q2’s $1 billion. VCs were drawn to drug delivery developers – nearly $96 million in venture funding was invested in the segment. (See Exhibit 4.) From this group Civitas Therapeutics Inc. closed the largest round, taking in $38 million from its Series B led by Bay City Capital to move into Phase III its lead compound CVT301, a levodopa aerosol for off episodes in Parkinson’s disease. [See Deal] Civitas aims to commercialize products delivered via its dry powder ARCUS inhaler. The start-up spun off from Alkermes PLC in 2010 and previously raised $25 million in Series A funds. [See Deal]
Exhibit 4
Drug Delivery Players Attract VC Funding in Q3 2013
|
Date |
Company |
Drug Delivery Technology |
Round # ($M raised) |
|
September |
Civitas Therapeutics |
ARCUS breath-activated dry powder inhaler |
Series B (38) |
|
August |
Icon Bioscience |
Verisome controlled-release intra-ocular delivery |
Series B (20) |
|
September |
Egalet |
Abuse- and tamper-resistant tablets that control the release of the drug via gradual erosion |
Series C (20) |
|
August |
Rani Therapeutics |
Formulation technology to make existing injectable biologics orally available while avoiding the degradation of the drug in the GI tract before it reaches the bloodstream |
Series B (10) |
|
August |
Highland Therapeutics |
Modified-release versions of approved and off-patent products, starting with amphetamine and methylphenidate |
Series A (6.7) |
|
September |
Aequus Pharmaceuticals |
Reformulating currently marketed drugs as transdermal products to lower dosing frequency, reduce first-pass metabolism side effects, and improve patient compliance |
Series A (1.2) |
Elsevier’s Strategic Transactions
Acquisitions
M&A activity in the third quarter 2013 was headed up by outlier Perrigo Co. PLC’s $8.3 billion acquisition of Elan [See Deal], representing 56% of the $14.8 billion total.) (See Exhibit 5.) The US generic drug maker plans to move operations to Elan’s Dublin, Ireland headquarters, which will give it tax advantages. (See (Also see "Perrigo’s Irish Move Sets Stage For International Boost, Net Revenue Gain" - Pink Sheet, 2 Aug, 2013.).) The transaction also hands Perrigo a 12-25% royalty stream from multiple sclerosis drug Tysabri (natalizumab), to which Biogen Inc. had partial rights under a 2000 arrangement [See Deal]. Biogen bought Elan’s remaining share for $3.25 billion earlier this year [See Deal]. (Perrigo’s acquisition put an end to a hostile five-month saga in which Elan rejected three bids from Royalty Pharma ranging from $11-13 per share, as much as $6.6 billion at the highest, plus CVRs. (See (Also see "Elan’s Saga Comes To A Close With Perrigo’s $8.6 Billion Bid" - Pink Sheet, 5 Aug, 2013.).)
Exhibit 5
Top Biopharma M&As, Q3 2013
|
Date |
Acquirer/Acquired (Business) |
Terms |
|
July |
Perrigo/Elan (Midsize European company; mostly neuro and immune disorder drugs) |
$8.3 billion: $16.17 per share ($6.25 in cash and 0.07636 in stock), a 57% premium; 41,376.5x sales |
|
September |
CFR Pharma/Adcock Ingram (branded generic medicines and anti-retrovirals) |
$1.26 billion in cash/stock combo determined after CFR completes financing: Adcock gets between 51-64.3% in cash (a minimum of ZAR37.49/maximum ZAR47.29 per Adcock share--a 27% discount at the top end) and between 35.7-49% in new CFR shares; 2.26x sales |
|
July |
Otsuka/Astex (cancer drug pipeline and discovery technology) |
$807 million: $8.50 in cash per share (a 40% premium); 9.71x sales |
|
July |
Cubist/Optimer (antibiotics) |
$767.4 million: $10.75 in cash per share (a 24% discount) and potential earn-outs of $243.6mm; 5.16x sales |
|
July |
Cubist/Trius (antibiotics) |
$748.2 million: $13.50 per share (a 17% premium) in cash and potential earn-outs of $96.5 million; 23.97x sales |
Elsevier’s Strategic Transactions
Sixteen of the 26 Q3 acquisitions had values exceeding $100 million with seven above the $500 million mark. At the top was Chile’s CFR Pharmaceuticals SA (specialty branded generics) takeover of publicly traded South African drug manufacturer Adcock Ingram Holdings Ltd. [See Deal]. The deal is worth a potential $1.26 billion in a cash and stock combination to be determined after CFR completes a 3 billion-share capital increase. The combined company will expand CFR’s existing emerging markets presence to more than 23 countries, including the additional territories of Africa and Southeast Asia, with added capabilities to export from these regions to Latin America and Southeast Asia. Adcock’s portfolio includes anti-retrovirals and hospital and critical care products.
In another generics play, spec pharma Akorn Inc. paid $591 million ($43.50 in cash per share – a 23% premium) for Hi-Tech Pharmacal Co. Inc. [See Deal], making Akorn the third-largest US generic ophthalmology drug company. Accounting for the majority of its revenues are Hi-Tech’s 57 generics (including versions of Merck & Co. Inc. and Johnson & Johnson eye drops) in a variety of dosing formulations. OTC drugs, health care products, and a small branded drug division make up its remaining sales. Akorn adds to its pipeline Hi-Tech’s 18 filed ANDAs and expects to increase its manufacturing capabilities through the addition of Hi-Tech’s two US plants. (See (Also see "Ophthalmology M&A Engine Keeps Roaring As Akorn Buys Out Hi-Tech" - Pink Sheet, 27 Aug, 2013.).) Another notable generics M&A was Endo International PLC’s Qualitest Pharmaceuticals’ $225 million buy of private generics company Boca Pharmacal LLC [See Deal]. (Endo bought the Qualitest division, along with its 600 generics, for $1.2 billion in 2010 [See Deal].) The Boca addition brings controlled substances, semi-solids and solutions, tablets, capsules, suspensions, and OTC vitamins and minerals across various therapeutic areas to Qualitest’s already-strong niche generics market segment. This follows a recent initiative by Qualitest to beef up its offerings through M&A, hoping to complete up to three deals in the $250-500 million range within the next 18 months. (See (Also see "Endo Makes First M&A Move Under New Regime, Building In Generics" - Pink Sheet, 28 Aug, 2013.) and (Also see "Endo Business Development On Track For Action" - Pink Sheet, 6 Aug, 2013.).)
Click here![]() to sign up for our free weekly newsletter to keep up on the latest published deals in Strategic Transactions.
to sign up for our free weekly newsletter to keep up on the latest published deals in Strategic Transactions.
Top Japanese drug and nutraceuticals firm Otsuka Pharmaceutical Co. Ltd. bought US cancer drug developer Astex Pharmaceuticals Inc. for $807 million [See Deal] in August, but not before some back-and-forth with shareholders, who initially opposed the transaction, claiming the offer price was too low and that the firm didn’t entertain enough competing offers. The deal gives Otsuka access to the US start up’s Dacogen (decitabine) – marketed by partners Eisai Co. Ltd. and J&J’s Janssen-Cilag International under a 2006 deal [See Deal] that provides Astex with a sales royalty stream between 20-30%. Otsuka also gets other potential royalty-bearing agreements with several Big Pharma partners, some mid-stage pipeline candidates in cancer, and a drug discovery platform technology. (See (Also see "Astex Finds A Suitable Home As Otsuka Subsidiary" - Pink Sheet, 5 Sep, 2013.).)
Mitsubishi Tanabe Pharma Corp., a division of top Japanese company Mitsubishi Chemical Holdings Corp., paid about $151 million (at $1.10 per share – a 29% premium) for a 54% stake in Canadian vaccine firm Medicago Inc. [See Deal], bringing its ownership to 60%. Medicago has a lead candidate in Phase II for H5N1, plus technology it claims can improve the efficacy, cost, and speed of vaccine production. The deal enables Mitsubishi to grow its vaccine presence to have an edge over fellow Japanese firm Takeda Pharmaceutical Co. Ltd. within this space. (See (Also see "Mitsubishi Tanabe Acquires Vaccine Development Partner Medicago" - Scrip, 12 Jul, 2013.).)
Cubist Pharmaceuticals Inc. had a pair of complementary acquisitions in July, getting its hands on public rivals in the antibiotics market. It bought Optimer Pharmaceuticals Inc. for $524 million in cash, plus CVRs that could amount to $243 million if all sales goals for Optimer’s marketed C. difficile drug Dificid (fidaxomicin) are met [See Deal]. Cubist also purchased Trius Therapeutics Inc., mainly for its Phase III antibiotic tedizolid (gram-positive and acute bacterial skin and skin structure infections), for $651.6 million in cash, plus up to $96.5 million in CVRs related to future net sales of tedizolid in the US, Canada, and Europe [See Deal]. (See (Also see "Next Up For Cubist: A Future That Includes Optimer, Trius" - In Vivo, 26 Aug, 2013.).) These two deals and four others were structured with earn-outs, a deal type that accounted for $2.7 billion in potential deal value of the Q3 M&A dollar total. (See Exhibit 6.) The Cubist M&As are the only two of the six deals in which the up-front payments exceed the potential earn-out amount; for all the other transactions, the earn-out is more, sometimes significantly.
Exhibit 6
Earn-Outs Could Add $2.7B To Q3 M&A Values
|
Date |
Acquirer/Acquired |
Potential Value ($M) |
Up-Front ($M) |
Earn-Out Amount ($M)/Condition |
|
July |
Cubist/Optimer |
767 |
524 |
243/net sales goals of Dificid (fidaxomicin) |
|
July |
Cubist/Trius |
748 |
651.6 |
96.5/future net sales goals of antibiotic tedizolid in US, Canada, and Europe |
|
August |
MedImmune/Amplimmune |
500 |
225 |
275/development-based goals |
|
July |
Bain Capital/ UK Department of Health’s Plasma Resources |
304 |
137 |
167/payable over next five years |
|
July |
Spectrum/Talon Therapeutics |
231 |
36 |
195/CVRs for future cash payments based on success of Talon’s Marqibo (topical menadione cream) |
|
July |
Ipsen/Syntaxin |
207 |
37 |
170/development and commercialization MS mostly linked to Phase II senrebotase (AGN214868; post-herpetic neuralgia, urinary incontinence, and overactive bladder) |
Elsevier’s Strategic Transactions
Midsize European company Ipsen bought private UK biotech Syntaxin Ltd. [See Deal], a specialist in botulinum toxin engineering with a pipeline of potential treatments for pain and cancer that work by blocking muscle spasms and tremors. Through the deal, Ipsen hopes to gain an advantage over competitor Allergan Inc., which currently leads the botulinum toxin market with its blockbuster Botox (onabotulinimtoxinA). Syntaxin’s bacterial protein engineering platform produces cell-specific therapies that inhibit cell secretion. (See (Also see "Ipsen Aims To Dominate In Botulinum Toxins With Syntaxin Buy" - Pink Sheet, 15 Jul, 2013.).) Ipsen wasn’t the only midsize European company that completed an acquisition; Recordati Industria Chimica & Farmaceutica SPA made twin third quarter purchases. In July it paid $48 million for the majority of Tunisian drug company Opalia Pharma SA [See Deal], and in September it bought Laboratorios Casen Fleet SLU (GI and OTC gynecological products) [See Deal] for $123 million, or more than twice the Spanish drug company’s 2012 revenues. Casen Fleet is the Madrid-based pharmaceutical business of US personal care products firm CB Fleet Co. Inc., which is divesting it to focus on the consumer market domestically.
Leading the pack of private biotech acquisitions during Q3 was AstraZeneca PLC’s MedImmune LLC, boosting its early-stage oncology pipeline with a $500 million buy of immune-mediating cancer therapy (IMT-C) developer Amplimmune Inc. [See Deal]. (See (Also see "MedImmune To Enhance Oncology Pipeline With Buyout Of Neighbor Amplimmune" - Pink Sheet, 26 Aug, 2013.).) This biotech’s main attraction is its late-preclinical anti-programmed cell death 1 monoclonal antibody (AMP514) with potential in cancer and infectious disease. Amplimmune also has partnerships with GlaxoSmithKline PLC, granting the Big Pharma exclusive worldwide rights to its then preclinical (now Phase I/II) candidate AMP224 for cancer and other diseases [See Deal], andDaiichi Sankyo Co. Ltd., which optioned the Phase I-ready autoimmune candidate AMP110 [See Deal]. Paying high prices to acquire private biotechs is becoming rarer and rarer as the IPO market thrives. Of the 10 private biotech acquisitions in the third quarter, just three were valued above $50 million.
Alliances
Of the 109 biopharmaceutical alliances completed during the third quarter of 2013, deals (for which the financial terms were disclosed) had a pre-commercialization value of at least $100 million. Leading the group was AstraZeneca’s $815 million link up with FibroGen Inc. (fibrotic diseases) [See Deal] for development and marketing rights to FG4592 in the US, China, and other countries — excluding some territories where Astellas Pharma Inc. holds rights under a 2006 deal [See Deal]. (See (Also see "AstraZeneca’s “Smart Risks” Include Sharing FibroGen’s Anemia Compound With Astellas" - Pink Sheet, 31 Jul, 2013.).) (See Exhibit 7.) FG4592 is a Phase III oral hypoxia-inducible factor inhibitor for anemia that is related to chronic kidney disease and end-stage renal disease. The new partnership will provide AZ with access to another late-stage drug candidate as it faces an approaching patent cliff; its heartburn treatment Nexium (esomezaprole) loses exclusivity in 2014 and cholesterol-lowering Crestor (rosuvastatin) in 2016. The Big Pharma’s anti-psychotic Seroquel (quetiapine) went off patent last year.
Exhibit 7
Top Biopharma Alliances, Q3 2013
|
Date |
Licenser/Licensee |
Potential Deal Value ($M)* |
Terms |
|
July |
FibroGen/AstraZeneca |
815 |
AZ pays $350 million uf; up to $465 million in dev. MS; sales MS; and royalties in the low 20% range for rights in the US, China, and other countries to FibroGen’s FG4592 for anemia associated with chronic kidney disease and end-stage renal disease. |
|
September |
Biogen Idec/Isis |
450 |
Biogen gets excl. rights to Isis’ antisense technology in the area of neurological disorders in exchange for $100 million uf, MS, license fees, and royalties. Amounts are linked to molecules’ characteristics; for antisense compounds Isis gets another $10 million MS with another possible $250 million in pre-commercial MS. For a small molecule or biologic Biogen pays a $5 million MS and another $85 million. |
|
September |
Galapagos/AbbVie |
405 |
AbbVie helps Galapagos discover, develop, and market cystic fibrosis therapeutics. AbbVie pays $45 million uf for an excl. ww license that excludes China and S. Korea, where Galapagos keeps rights; Galapagos also has co-promotion rights in Belgium, the Netherlands, and Luxembourg. AbbVie will also pay up to $360 million in dev. and reg. MS; sales MS; and double-digit royalties. |
|
September |
Coherus BioSciences/Baxter International |
246 |
Baxter pays $30 million uf and up to $216 million in dev. and reg. MS for European, Canadian, Brazilian, and other excl rights to Coherus’ biosimilar formulation of etanercept for autoimmune diseases. |
|
July |
Immunocore/GlaxoSmithKline |
212 |
GSK licenses rights to Immunocore’s ImmTACs, which interact with several targets that haven’t been successfully addressed by traditional antibodies. GSK pays $212 million in preclinical MS for all the targets, up to $298 million in clinical and commercial MS per product, and up to double-digit royalties. |
*Potential Deal Value is the sum of up-front fees/equity plus pre-commercialization money.
Elsevier’s Strategic Transactions
Unlike Q2, in which early-stage drug candidates represented most of the in-licensed assets, the majority of the third-quarter alliances favored already marketed products. (See Exhibit 8.) Amgen Inc. led with its agreement for Servier SA’s ivabradine, which is sold in over 100 countries across the world as Procoralan for chronic heart failure and stable angina in patients who are experiencing increased heart rates [See Deal]. Amgen provided $50 million up front, with the potential for milestones and royalties. Also tucked into the deal was the exclusive option to develop and market in the US Phase II S38844 for cardiovascular diseases. The deal provided Servier the option for European rights to omecamtiv mecarbil for heart failure in systolic dysfunction patients; the drug is in Phase II under terms of a 2007 collaboration between Amgen and Cytokinetics Inc. [See Deal] (See (Also see "Double-Jointed Partnership With Servier Could Jumpstart Amgen’s CV Portfolio" - Pink Sheet, 11 Jul, 2013.).)
Exhibit 8
Marketed Drugs: The Clear Leader In Biopharma Deals, Q3 2013
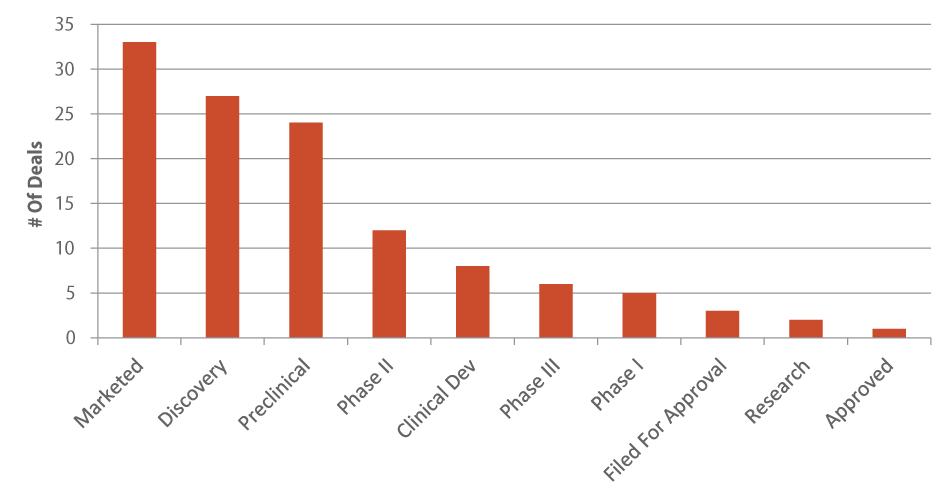
NOTE: If alliances had more than one licensed drug candidate, they were counted multiple times based upon their respective phases.
Elsevier’s Strategic Transactions
Glybera (alipogene tiparvovec), the first gene therapy ever approved (in November 2012), was also partnered during the third quarter. Italian pharmaco Chiesi Farmaceutici SPAlicensed exclusive rights from Dutch biotech start-up uniQure NVin Europe, Brazil, Mexico, Pakistan, Turkey, Russia, the CIS, and China [See Deal]. Glybera is indicated for lipoprotein lipase deficiency, a rare disorder that interferes with the breakdown of fats in the body. Much like the Amgen deal, Chiesi also gained co-development rights to uniQure’s Phase I/II AMT060 hemophilia B gene candidate. Chiesi took an $18 million equity stake in uniQure and provided $21.8 million in cash up front. In addition, Chiesi will cover 50% of the remaining development expenses for AMT060 and pay between 20-30% royalties on both products. (See (Also see "uniQure Gets Its Groove On: An Interview With CEO Jörn Aldag" - Pink Sheet, 22 Jul, 2013.).)
Discovery-stage alliances came in a close second (27 such deals were completed versus 33 for marketed drugs), with several notable partnerships featured in the top five alliances this quarter. In their fourth coupling since the beginning of 2012, Biogen Idec and antisense-focused Ionis Pharmaceuticals Inc. allied in a deal with a pre-commercialization value of $450 million focused on treatments for neurological disorders [See Deal]. (See (Also see "Isis Builds On Platform Strategy With Fourth Biogen Tie-Up" - Pink Sheet, 9 Sep, 2013.).) Another large early-stage deal involved Abbott Laboratories Inc. spin-off AbbVie Inc. linking with Galapagos NV to discover fibrosis therapies [See Deal]. Monies could total $405 million if all milestones are met. (See (Also see "Two Days, Two Deals: AbbVie To Partner (Again) With Galapagos In Cystic Fibrosis" - Pink Sheet, 24 Sep, 2013.).) AbbVie was also busy penning a deal that capitalizes on its rheumatology expertise, teaming up with Ablynx NV to develop and market ALX0061, a Nanobody for inflammatory diseases [See Deal]. AbbVie will contribute $175 million up front for clinical trials, up to $665 million in development, regulatory, commercialization, and sales milestones, and double-digit tiered royalties. Ablynx will finish up Phase II and at that point AbbVie can opt to license the compound and pick up the remainder of development and global marketing activities. (See (Also see "Nanobody Specialist Ablynx Bags Global IL-6R Deal With AbbVie" - Pink Sheet, 23 Sep, 2013.).)
Product divestures — many of which involved generics firms — also left their mark during Q3 2013. Breckenridge Pharmaceutical Inc. paid $20 million up front in cash for some Cypress Pharmaceutical (an absorbed division of Pernix Therapeutics Holdings Inc. [See Deal]) assets, including 11 filed ANDAs, some in-process ANDAs, and seven formerly marketed generics [See Deal]. Pernix divested the therapeutics as a result of consolidation after two acquisitions in just one year’s time: the aforementioned Cypress and specialty firm Somaxon Pharmaceuticals Inc. [See Deal].
Purchases were also made in order to close several notable acquisitions. Before Mylan NV can buy Strides Shasun Ltd.’s Agila Specialties Pvt. Ltd. in a deal worth $1.6 billion [See Deal], the US Federal Trade Commission is requiring the companies to divest 11 generic injectables for cancer, autoimmune and infectious diseases, cardiovascular conditions, poisoning, and a surgical anesthetic to JHP Pharmaceuticals LLC, Sagent Pharmaceuticals Inc., Gland Pharma Ltd., and Intas Pharmaceuticals Ltd. [See Deal]. The transaction is the fourth-largest divestiture due to an acquisition in generics history — behind Teva Pharmaceutical Industries Ltd.’s buy of Barr Pharmaceuticals [See Deal],Watson’s purchase of Allergan PLC [See Deal], and Teva picking up Ivax [See Deal]. Likewise, to complete its merger with Warner Chilcott PLC [See Deal], Actavis had to sell four generics in Q3 to Amneal Pharmaceuticals LLC, including several oral contraceptives and risedronate for osteoporosis [See Deal].
Cancer drugs were by far the most sought-after in the third quarter, with about a third of these deals involving Big Pharmas. (See Exhibit 9.) Roche and Bayer AG completed two cancer deals each. Wilex AG’s Heidelberg Pharma GMBH division granted Roche rights to its antibody-drug conjugate (ADC) technology; Roche got the option to develop and market any resulting products [See Deal]. This is Roche’s first ADC alliance although its subsidiaries, Genentech Inc. in particular, have already penned ADC deals with firms that include Seattle Genetics Inc. [See Deal], PDL BioPharma Inc. [See Deal], and Spirogen Ltd. [See Deal]). In its other oncology tie-up, Roche took exclusive worldwide rights to Inovio Pharmaceuticals Inc.’s preclinical DNA vaccines — INO5150 for prostate cancer and INO1800 for hepatitis B [See Deal]. It’s been three years since Roche’s last vaccines collaboration; in late 2010 the Big Pharma secured rights to immunology programs (including those for cancer vaccines) that were co-developed with the Baylor Institute for Immunology Research [See Deal].
Bayer, too, was active in oncology licensing deals. It paired with the Broad Institute to discover and develop compounds aimed at cancer genome alterations; Bayer has the exclusive option to programs once they’ve reached preclinical testing [See Deal]. Bayer also teamed up with Compugen Ltd. for the second time this quarter [See Deal]. This time around the Big Pharma gets worldwide rights to cancer antibodies that target two immune checkpoint regulators key in immunosuppression.
Exhibit 9
Big Pharma At The Heart Of Cancer Biopharma Partnerships, Q3 2013
|
Date |
Big Pharma/Partner |
Subject Of Alliance |
|
July |
Novartis/Life Technologies |
Novartis to use Life Tech’s DynabeadsDC3/CD28 CTS to develop immunotherapeutics, specifically chimeric antigen receptors for cancer. |
|
July |
MedImmune/Kolltan |
Kolltan gets rights to MedImmune’s preclinical HER-3 inhibitor, linked to breast, lung, ovarian, and colon cancer. |
|
July |
J&J’s Janssen Biotech/Amphivena Therapeutics |
Janssen invests in Amphivena, gets option to outright acquire the firm, which is focused on bispecific TandAb antibodies for hematological conditions. |
|
August |
Bayer/Compugen |
Bayer gets rights to Compugen’s cancer antibodies involved in immunosuppression. |
|
September |
Boehringer Ingelheim/Diaxonhit |
BI will develop Diaxonhit’s splice variant targets. |
|
September |
Bayer/Broad Institute |
Partners will work on oncogenomics for compounds that target cancer genome alterations. Bayer has excl. option to programs once they reach preclinical studies. |
|
September |
Roche/Heidelberg Pharma |
Roche has rights to Heidelberg’s ADC technology, which Roche will use with its antibodies. |
|
September |
AstraZeneca/Merck |
AZ gets access to Merck’s MK1775, a Phase IIa compound for P53-deficient ovarian cancer. |
|
September |
Roche/Inovio |
Roche gets excl. ww rights to the DNA vaccines INO5150 for prostate cancer and INO1800 for hepatitis B. |
Elsevier’s Strategic Transactions