Can MicroPort Master Orthopedics?
Executive Summary
China’s MicroPort Scientific paid $290 million in cash for Wright Medical’s hip and knee business, which has a pipeline of products – including FDA approved implants – ready for sale in China’s growing orthopedics market. The acquisition pits MicroPort against multinationals that previously held an edge selling implants to Chinese patients and doctors who seem to favor devices that carry approval from the US FDA.
- MicroPort Scientific Corp. paid $290 million in cash for Wright Medical’s hip and knee business, which holds miniscule market shares in the US, Europe, and China.
- But Wright Medical did come with a pipeline of products – including FDA-approved hip and knee implants – ready for sale in China’s growing orthopedics market.
- The acquisition pits MicroPort against multinationals that previously held an edge selling implants to Chinese patients and doctors who favored devices that carry approval from the US Food and Drug Administration.
- But MicroPort isn’t stopping there. The company has plans to leverage the former Wright recon’s reach into the US and Europe, using existing sales channels to sell orthopedic implants and other products, including its latest drug-eluting stent.
- With a supply of familiar implants and an eagerness to disrupt existing sales channels, MicroPort will try to seize market share in China, Japan, Europe, and the US.
Three years ago, Ted Davis was doing what any good business development officer at a US-based ortho company should do. His employer, Wright Medical Group NV, was trying to sell hip and knee implants in China. “We were growing at a pretty decent rate,” Davis recalls, but the company wanted to amp things up. So he was taking meetings, not really looking for partners but just trying better to understand the lay of the land. In his search, several signs kept pointing to MicroPort Scientific Corp., which had no real experience in orthopedics sales. “Several bankers told me, ‘Look, you need to meet with these guys,’” he recalls. “‘They are very dedicated to trying to develop a hip and knee strategy within China.’”
Boy, were they right. Three years after Davis had the meeting, he’s the CEO of MicroPort Orthopedics Inc., a new division of the Shanghai-based company. The new orthopedics venture combines Wright’s hip and knee business, which held only a small bit of market share in the US and Europe, with MicroPort’s nascent line of trauma and spine products that it sold in China. MicroPort Orthopedics stands as the first Chinese company to sell hip and knee implants with regulatory approval in international markets, including the US. MicroPort, which is already a market leader in several device sectors in China, now has the armament necessary to compete against multinational players in China in orthopedics. (See (Also see "MicroPort’s Wright Bid Creates New Ortho Player" - In Vivo, 25 Jul, 2013.).)
In recent years, several multinational companies have built inroads into China’s orthopedics market. Stryker Corp. and Medtronic PLC bought themselves significant access with the recent acquisitions of two of China’s largest players – Trauson Holdings Co. Ltd. and China Kanghui Holdings. [See Deal][See Deal] Companies like Smith & Nephew PLCand Zimmer Biomet Holdings Inc. have spent years establishing their own presence in China’s markets, using their FDA-approved implants to pitch their wares to China’s wealthier hospitals and patients.
China is a market where multinationals can still realize significant growth selling hip and knee implants to an aging population. Large joints, as they’re called, have for the most part been moribund in the US and Europe, so China’s double-digit growth balanced the slowdowns in older markets. But the customers are increasingly difficult to reach as new business emerges in more remote portions of the country. (See (Also see "Kanghui: A New Strategic Opens The Door To Orthopedics In China" - In Vivo, 1 Oct, 2011.).)
For MicroPort Orthopedics, China – and its potential for growth – actually might be the low hanging fruit. Founded in 1998, MicroPort already sells several lines of devices including endovascular grafts, neurovascular tools, and electrophysiology. Jonathan Chen, vice president of international business and investor relations for MicroPort Scientific, notes that MicroPort is the top seller of each line in China.
Three years ago, MicroPort leadership saw orthopedics as a gap in its offerings. The company responded by creating its own line of trauma and spine products. Two years ago, in fact, MicroPort secured 510(k) approval for its Reindeer locking plate system, the company's first approval to sell in the US. But the absence of hip and knee implants kept MicroPort from competing with multinationals.
Growth From Within
Founded in 1998, the company has grown largely through research and development, building its own line of products to take on multinationals. Founder Zhaohua Chang, an engineer and now the company’s executive director, built the company around a drug-eluting stent. A decade ago, US and European multinationals had a lock on the market. But MicroPort introduced its own DES and eventually seized the top spot in selling DES in China. The company spends between 15% and 18% of its revenue on research and development, about twice the industry average, and had been able to create a broad pipeline of products covering many therapeutic sectors and devices from endovascular grafts to treat abdominal aortic aneurysms to electrophysiology devices, including a three-dimensional (3-D) navigational system used to treat atrial fibrillation. The firm also has its own line of neurovascular stents used in neurosurgery.
MicroPort, undoubtedly, could have developed its own line of hip and knee implants. However, the orthopedics sector presents interesting challenges. The first is the fact patients and surgeons in China tend to favor devices carrying approval from the US Food and Drug Administration, so if MicroPort wanted to compete with multinationals it may have had to obtain approvals in both the US and China. MicroPort faced a similar challenge in the US where surgeons and hospitals prefer prefer using implants manufactured in the United States. Knee and hip implants sometimes have to last a lifetime, so surgeons aren’t willing to use an implant from an unfamiliar source.
Instead of building from within, MicroPort agreed to pay $290 million in cash for Wright’s hip and knee implants. [See Deal] The acquisition brought an end to Wright Medical’s often troubled connection to the hip and knee industry. The most recent problem arose in 2010 when the US government filed a criminal complaint against the company alleging that it had used consulting agreements with surgeons to sell implants. The charges cost the former CEO his job, opening the door for current CEO Robert Palmisano to take charge. (See.)
The marriage of an FDA-approved line of implants and a homegrown sales force with existing connections in China’s hospitals and surgical suites immediately establishes MicroPort as a player in China’s orthopedics market.
The marriage of an FDA-approved line of implants and a homegrown sales force with existing connections in China’s hospitals and surgical suites immediately establishes MicroPort as a player in China’s orthopedics market. Wright, while holding only a meager share of China’s market, was able to obtain regulatory approval for three-quarters of its hip and knee implants. So MicroPort now is positioned to compete with multinationals from Europe and the US that previously were the only companies capable of offering FDA-approved implants in China. It also has 120 employees and 60 distributors to utilize to sell in China. “We will be one of the very few, if not only domestic companies that has this portfolio of hip and knee products, spine products, trauma products,” Chen says. “So we feel that we can leverage our market leadership in the China market” to ramp up sales almost immediately.
In his meeting three years ago, Davis couldn’t have foreseen how his path would cross MicroPort’s again. But Chen, who was not with MicroPort at the time of the meeting, says the company was keeping an eye on Wright three years prior to the purchase. It’s easy to understand why. MicroPort executives had two principal objectives: to enter the orthopedics industry in China and to diversify the firm’s revenue sources. Wright’s orthorecon program fit MicroPort’s needs nicely. It was also affordable and clearly available.
One Company, Two Paths
Palmisano executed the vision he began etching out when he joined Wright in 2011. He had already drawn a clear line down the middle of the company’s Arlington, TN, headquarters. Wright would be an extremities and orthobiologics company. Actually, Wright had been following a similar strategy for years under Palmisano’s predecessor. (See (Also see "Is Wright Medical Back on Track?" - In Vivo, 1 Jul, 2009.).) But Palmisano made the commitment to extremities total and complete. Hips and knees, while a significant revenue maker, were out.
Palmisano structured the company into two distinct divisions, and one of the divisions – hip and knee – clearly was not part of Wright’s long-term vision. Davis, who had helped build out Wright’s extremities line, was named head of the hip and knee business. Palmisano challenged him with helping the division find a new future.
Davis says dividing Wright into two distinct parts clearly “helped create some visibility of what we had.” Wright was approached by a few suitors, but no deal was done. Meanwhile, MicroPort wanted to enter China’s orthopedics market. MicroPort and Wright’s recon group fit like puzzle.
Wright, while selling only $3 million in hip and knee implants in China last year, had successfully pushed two-thirds of its hip and knee portfolio through China’s regulatory channels. Davis says MicroPort is helping to get the rest of the products approved. “Their credibility with the CFDA is great,” he says. “They have been able to fix things that were stuck for us just because they know the agency.” China only accounted for roughly 1% of Wright’s hip and knee sales, so sales in China should improve almost immediately. “We should be able to do $3 million just by waking up and showing up at work every day,” Chen jokes.
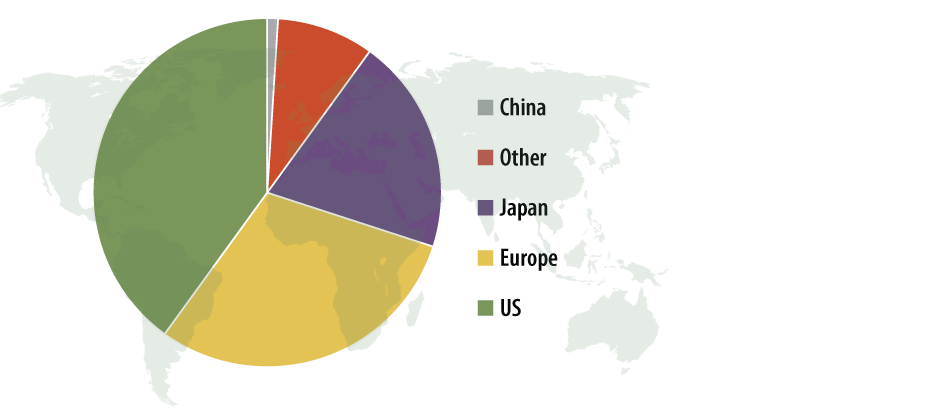
The acquisition also immediately diversified MicroPort’s sales stream. Prior to the acquisition, 80% of the firm’s sales came from drug-eluting stents, primarily in China where it’s first in the market. The addition of the hip and knee products immediately brought that percentage down to 40%, Chen says. Wright’s ortho recon business also opens doors to other markets. Roughly 40% of Wright OrthoRecon’s $250 million sales came from the US. Europe accounts for 30% with Japan following with another 20%. MicroPort intends to continue selling orthopedic implants in all those markets, but it also could use Wright’s former international offices to sell other products. For example, MicroPort intends to export its latest stent, FireHawk. Chen, in a presentation at the 2014 JP Morgan Healthcare Conference in January, said the company has filed for CE mark and could launch the stent in Europe by the end of this year. (It has no plans to launch in the US. Chen says clinical hurdles are too expensive.)
Exhibit 1
MicroPort Orthopedics’ New Global Reach*
*Based upon number of Wright Medical’s Recon Group.
MicroPort Orthopedics
Chen says the move into international markets is another part of MicroPort’s DNA. Senior executives at the company share a similar profile; they’re born in China but were educated in the US and took jobs at US device companies for a decade or more. “They spent the better part of their professional career getting training first in Western companies and learning the product development processes, the R&D processes, and have brought this knowledge base back to China and MicroPort,” he explains. "Even though our company is based in Shanghai, it’s managed like a Western company.”
Changing The Game
Given its success in selling other devices in China, MicroPort likely will hit the ground running selling orthopedics in China. Chen says the challenge for Chinese companies has been obtaining technology “comparable to what Western multinational companies are able to produce.” With Wright’s hips and knees, the question is going to be who has the best distribution channel within the China market. “We feel that since China is our home market, we should have a very compelling strategic advantage on the distribution side versus a Western multinational company trying to access a domestic China market,” Chen explains. MicroPort’s challenge will come in selling into international markets.
Davis says MicroPort Orthopedics won’t push too deeply into the emerging markets outside of China (Latin American is a possibility), but the company will look to grab larger market share in Japan, the US, and Europe. The push to grab market share in Europe and the US might seem quixotic, but the company sees an opportunity to disrupt these markets. First, Chen says hip and knee businesses in the US and Europe will benefit immediately from not being neglected any longer. For the past half-decade, Wright’s hip and knee programs were overshadowed by the push into extremities. It’s easy to forget the company was once an innovator in hips and knees. For example, a decade ago, Wright and Stryker were the first companies to receive FDA clearance to sell ceramic hip implants in the US.
Chen points out that Wright was the first to launch the modular hip, metal on metal, and metal on ceramic. The company also introduced early versions of cutting blocks used by surgeons to make implantation more precise. But even with the innovation, Wright traditionally had trouble breaking the hold of the larger companies. The years of neglect only dulled the brand. Analysts covering the orthopedics industry say the company’s product line isn’t known by surgeons for having any distinctive features. “They’ve viewed by surgeons as being okay, not great,” says one analyst.
Chen says MicroPort Orthopedics will turn the spigot back on. “Our message to the employees who are coming over is that we want to turn the innovation engine back on for this ortho recon business,” he says. “Wright Medical has a long history in the US orthopedics market. They’ve been in business for over 60 years. And at one point, prior to them going into extremities, Wright Medical was known as being an innovator for the hip and knee markets.”
Chen says MicroPort will highlight the Evolution knee replacement system, which Wright released in 2010. The knee offers more sizing options and a medial-pivoting posterior stabilized option. “We’re consistently hearing from the surgeons that this is the best-in-class knee in the market,” Chen says. “So we ask, ‘Well, if that’s the case, then how come it hasn’t done better in terms of revenue?’ The answer is, ‘Well, you haven't really gone out and told people about it.’” Chen is optimistic that with additional marketing dollars MicroPort will be able to increase the penetration of the knee product.
Chen also called out the SuperPath hip replacement system, which can replace a hip without dislocating it, sometimes through a 3-inch incision. “Patients literally can go into surgery and be in a position to walk out of the hospital the same day,” he says. “The rehab is usually several weeks. But we have patients getting off the table and walking out the door, literally the same day of this minimally invasive procedure.”
A Tough Road
Joanne Wuensch, medical analyst at BMO Capital, says the increased resources will help, but the deck continues to be stacked against smaller implant manufacturers. (Wuensch and other analysts cover Wright and other orthopedic companies but don’t cover MicroPort.) “There are so many hip and knee contractors out there or dual source agreements that are already inked,” she notes. MicroPort will need to push hard to find a way into hospitals. But even existing players are struggling to differentiate themselves in a market that’s converting implants into lower cost commodities.
Hip and knee implants themselves haven’t traditional inspired drastic innovation. But the push toward improving the fit and balancing of implants will likely produce some interesting story lines in coming years. Large joint makers already have introduced so-called personalized implants design to fit different anatomies. (See (Also see "At AAOS Knee Implant Makers Pitch Better Fit, Precision" - Medtech Insight, 26 Apr, 2013.).) More and more, large joint makers are striving to distinguish themselves and their implants in what’s increasingly becoming a commodities business. Stryker, for example, acquired surgical robot maker MAKO Surgical Corp. last year. [See Deal] (See (Also see "Will Stryker’s MAKO Purchase Bring Disruption To The Ortho Industry?" - Medtech Insight, 28 Oct, 2013.).)
MicroPort’s initial edge may come through cost savings, both for itself and its customers. MicroPort Orthopedics will continue to manufacture hip and knee implants in Tennessee. In fact, the division has taken over the entire Wright Medical headquarters in Arlington. But the company likely will move surgical instrumentation to MicroPort’s China facilities. Chen says, “We feel that over time we can probably reduce the cost by as much as 50%.”
In the US and Europe, MicroPort might use price to get a foot in the door. The company is picking up Wright’s attempt to undercut competitors on pricing, particularly in the US where hospitals are struggling to operate within the financial bounds of the Affordable Care Act. MicroPort rebranded “Wright Direct,” a campaign to cut costs by selling implants and tools with only the limited support of a sales rep, as Implant Partners. Chen explains, “Hospitals definitely want to manage their costs but it will take a lot of effort to get buy-in from everybody” including surgeons and insurance companies.
If MicroPort gains traction, Davis anticipates increasing the size of his sales team in the US.
If MicroPort gains traction, Davis anticipates increasing the size of his sales team in the US. Davis concedes that Wright lost some sales personnel during the transition, but that had already been an issue before the sale as Wright moved away from large joints. Now, Davis says, MicroPort Orthopedics is going into growth mode, pouring money back into surgeon education and marketing. “We have an advantage as the little guy,” he says. “As the market changes, a little guy can attract sales folks that want to be in a place where they make more of a difference. And frankly, we probably can have a better economic argument for them.”
As price constraints get tighter, MicroPort’s chances for success might improve. But large joint makers can also compete easily on price if challenged. Matt Miksic, managing director and senior research analyst at Piper Jaffray, says a company will have a difficult time picking up market share based on price alone. “If Zimmer woke up and said, ‘Our new strategy is to sell our implants for 20% less and use that as leverage to get new business,’ it might not go over so well with investors, but it would be disruptive. They could switch some surgeons.” Miksic says European hospitals may be more amenable to new pricing structures, but many of the pressures will be the same.
Japan is of particular interest. MicroPort’s $290 million offer for the recon division included a $200 million loan from one of its larger investors, Otsuka Medical Devices Co. Ltd. Chen says the low interest loan gave MicroPort the capital necessary to do the deal. Japan-based Otsuka now has an interest in an orthopedics recon company ready to make an aggressive push into its native land.
The next few years will be transformative for MicroPort and the orthopedics industry. China’s growing health care economy will continue to draw multinationals to the country where MicroPort could provide an effective counterbalance. In addition to adding orthopedics, MicroPort signed a joint venture with Italian Sorin Group SPA to sell cardiac rhythm management products in China. Together, MicroPort and Sorin committed $20 million to the venture, with the former taking a 51% stake. MicroPort intends to first sell but ultimately manufacture Sorin’s pacemakers in China.
In the US, the orthopedics industry is bound to undergo some consolidation as implant manufacturers vie for a larger piece of a limited market. The impact of MicroPort on that scrum remains to be seen. The company will have to overcome initial concerns from surgeons and patients who don’t easily accept change or new names. But if MicroPort can bring resources to revive the moribund Wright hip and knee brands, the company likely will become a fixture on the global orthopedics scene.