One Size No Longer Fits All: The Personalized Medicine Trial Landscape
Executive Summary
Success rates are higher for clinical trials that incorporate selective pharmacogenomics and pharmacogenetics (PGX) biomarkers, according to data from Informa's Trialtrove. Given the challenges of designing and administering trials to secure regulatory approval in areas of unmet medical need, the data provide some basis for optimism in realizing the promise of targeted, personalized therapies that improve health outcomes for individual patients.
- Advanced, genetically based testing and diagnostic tools support success in drug clinical trials, according to Informa's Trialtrove.
- Phase III programs with PGX biomarkers scored a significant 76.5% success rate, while Phase III programs without these selective biomarkers had a rate of only 55%.
- The data suggest that PGX tools have applicability to address one of the biggest challenges in clinical trial management today: patient recruitment and enrollment.
- Further, incorporating PGX biomarkers in trials could boost prospects for regulatory approval.
Although the standard of care for a disease (when one exists) generally has a well-established track record, it’s a one-size-fits-all remedy that may not be the best option for some patients. Medicine is entering a new era of personalization and the number of trials targeting specific subsets of patient populations continues to rise. The definition of “personalized medicine” or “precision medicine” varies, and can refer to an approach that incorporates insights on environmental and behavioral factors, in addition to a patient’s biology or genome, while informing disease treatment or prevention.
Some have a more specific view – personalized medicine leverages genetic profiles of patients to create tailored, more targeted interventions to better treat or prevent their conditions. This analysis will hone in on this arm of personalized medicine, exploring the current state of clinical research incorporating this strategy into drug development, and the potential effect of these approaches on the success of such trials.
The Course Of Clinical Research With Biomarkers
Biomarkers, genomic and non-genomic, have been used within clinical trials to facilitate drug development for different purposes, such as to serve as indicators of a drug’s toxicity or efficacy or to identify specific patient populations for targeted treatments. The latter has been enabled by the accomplishments of the Human Genome Project (1990–2003) – a complete sequence and map of all the genes in the human body.
With this better understanding of the human genome following the end of the project in 2003, the proportion of trials using pharmacogenetic or pharmacogenomic (PGX) biomarkers to target specific patients continually increased. Initially, trials that utilized PGX analyses to drive patient selection into a trial or into a cohort within a trial (patient stratification) hovered between 17% and 22% of all clinical research with biomarkers. From 2008 onward, the proportion continually increased until it comprised over half of biomarker research in 2016 captured by Informa Pharma Intelligence's Trialtrove. (See Exhibit 1.)
Exhibit 1
Phase I–IV Trials Using Biomarkers By Trial Start Year, 2003–2016
Trialtrove | Pharma Intelligence, 2017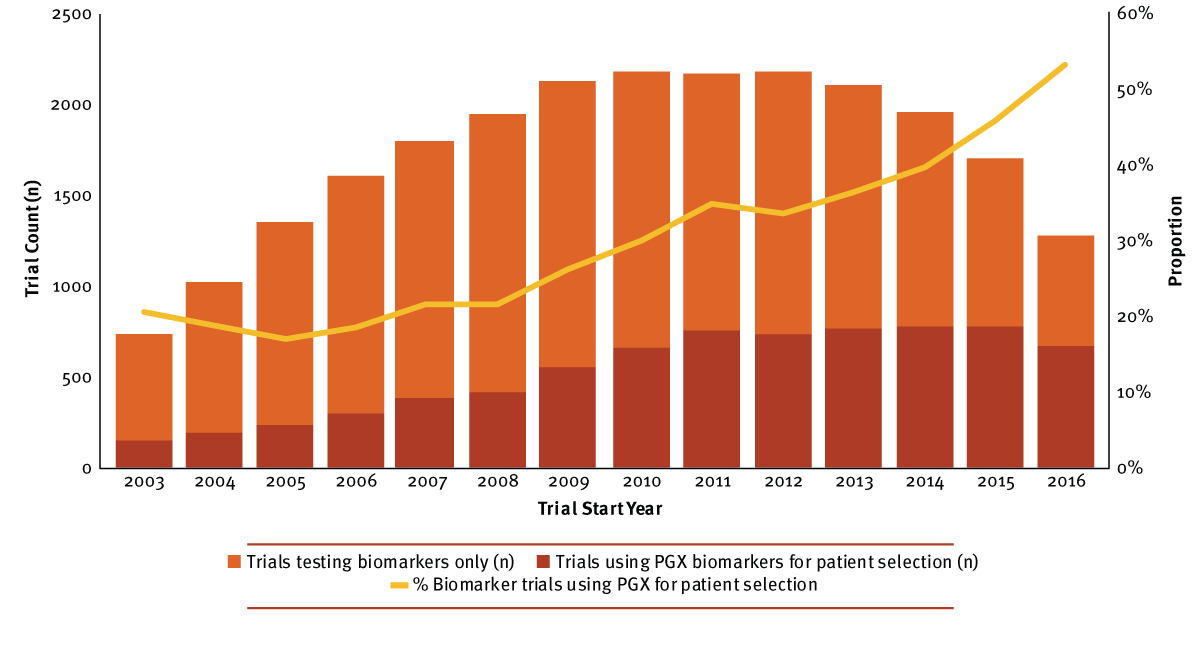
The largest percentage of clinical trials selecting or stratifying patients by PGX biomarkers took place in early- to mid-stage research. Due to their less frequent occurrence, trial hybrids were included in counts for the more advanced phase throughout this analysis (i.e., Phase I/II trials were counted as Phase II). Between 2003 and 2016, Phase II biomarker trials most frequently used PGX analyses for patient selection, followed by Phase I (36% and 31%, respectively). Only 29% of Phase III biomarker trials leveraged this strategy; however, interest has increased markedly in recent years, with the proportion jumping from 20% in 2000 to 53% in 2015, then 57% in 2016. PGX-informed patient selection was least common in Phase IV biomarker research (13%). Seemingly, postmarketing biomarker trials appear to be more focused on identifying novel biomarkers to indicate or predict therapeutic efficacy and/or drug toxicity, and not informing patient selection with genomics. (Data not shown.)
Since the completion of the Human Genome Project, more than 1,800 disease genes have been discovered, and over 2,000 genetic tests for human conditions now exist. However, not all diseases or conditions are associated with particular genetic mutations, and many are unable to reap the potential benefits of this approach. Oncology has seen a tremendous amount of activity, as well as benefit. Because cancer is a genetic disease, caused by errors in DNA that lead to uncontrolled growth of cells, it has been a frontrunner in incorporating targeted approaches into drug development activities.
Oncology
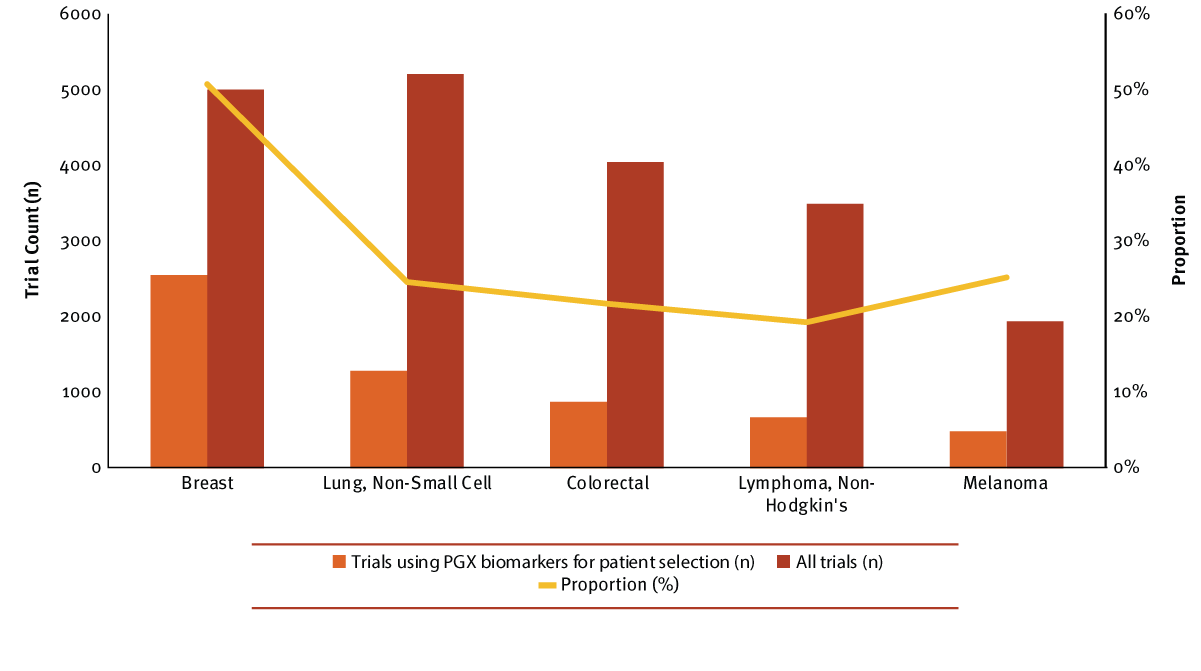
The vast majority of research with PGX-driven patient selection does indeed involve oncology indications; a whopping 90% of these trials were for cancer research. Overall, breast cancer led with the largest number of trials selecting patients based on PGX biomarkers, comprising 35% of all PGX biomarker selection trials. Non-small cell lung cancer (NSCLC) trails behind with nearly half the number of trials, followed by colorectal, non-Hodgkin’s lymphoma (NHL) and finally, melanoma. Breast cancer also holds the largest proportion of trials leveraging this strategy, and over 50% of all breast cancer research incorporates a genomic marker for patient selection. In contrast, the other top cancers use this personalized approach in 16% to 25% of their trials. (See Exhibit 2.)
Exhibit 2
Top Oncology Indications Selecting Or Stratifying Patients By PGX Biomarkers
Trialtrove | Pharma Intelligence, 2017
For each of the top five cancers, the number of PGX biomarker selection trials starting each year grew, although some appear to have declined in recent years. Trial counts for nearly all cancer types seemed to decrease in 2016; however, this could partially be attributed to reporting bias rather than a true decrease in trial activity. The exception was NHL, which had an uptick of 13 trials between 2015 and 2016. Year-on-year, breast cancer consistently held the top ranking for the number of PGX selection trials initiated, while the other four had similar levels of activity until 2008 when NSCLC and colorectal broke from the pack. NSCLC trial starts continued to rise, outpacing colorectal, until NSCLC consistently held its second place ranking. The trajectory of activity for colorectal initially positioned the cancer type for second place until its peak in 2010 and subsequent decline to levels similar to the trial activity of NHL and melanoma. One likely contributor to the rising levels of genomic-driven cancer research is the establishment of the Cancer Genome Atlas (TCGA) program, a collaborative effort from the National Cancer Institute (NCI) and National Human Genome Research Institute (NHGRI) to generate comprehensive genomic maps of 33 types of cancer. Although the program is nearing its end in 2017, the efforts of the TCGA, and other similar initiatives, will continue to inform targeted drug development and enable identification of additional therapeutic targets based on the expanding number of known genomic characteristics of various tumor types.
Non-oncology
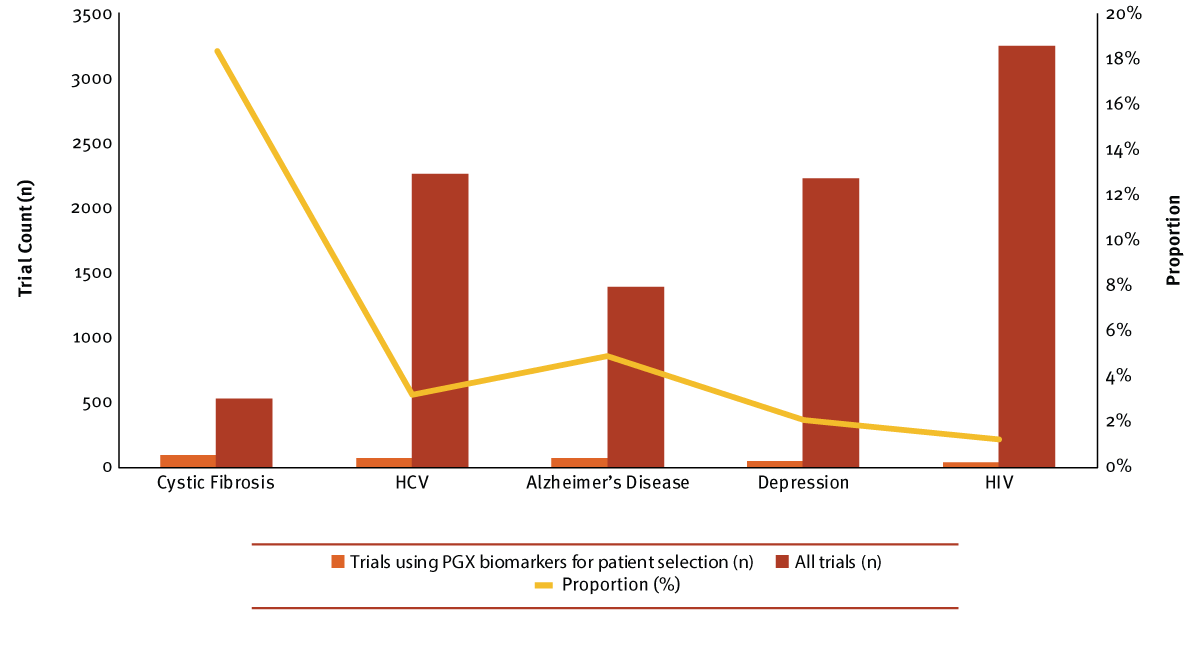
On a much smaller scale, non-oncology indications have also explored targeted approaches, primarily split between the therapeutic areas of CNS (central nervous system) and infectious disease. However, the leader overall and in recent years was an autoimmune disease, cystic fibrosis. Cystic fibrosis is the only non-oncology indication with a proportion of PGX biomarker selection trial activity similar to the top cancers, and 18% of all cystic fibrosis trials included this approach. In sharp contrast, the remaining four diseases – HCV, Alzheimer’s disease, depression and HIV – only selected patients with PGX biomarkers for 1% to 5% of all their clinical research. (See Exhibit 3.) (Also see "Cystic Fibrosis: Successes, Opportunities And Challenges" - In Vivo, 22 Feb, 2017.)
Exhibit 3
Top Non-oncology Indications Selecting Or Stratifying Patients By PGX Biomarkers
Trialtrove | Pharma Intelligence, 2017
While cystic fibrosis was the overall lead, the disease only attained this position in recent years, following the FDA approval of Vertex Pharmaceuticals Inc.'s Kalydeco (ivacaftor) in 2012 to treat patients with specific mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This uptick in trial activity for cystic fibrosis was largely driven by trials including Kalydeco, or invacaftor containing fixed-dose combinations. Prior to that, the top position oscillated among the top five non-oncology diseases, except for a brief period when HCV activity spiked in 2010 and 2011. In general, activity for indications outside of oncology has generally been minimal for most in recent years, rarely exceeding more than 10 trial starts in a year. Despite potential genetic causes and links for some non-oncology indications, targeted approaches have not been as heavily pursued. (Also see "Companion Diagnostics: The Expanding Reach Of Personalized Medicine" - In Vivo, 14 Mar, 2017.)
Key Destinations And Active Players
As of February 2017, Trialtrove captured 4,133 active PGX trials, defined as those that are currently planned or ongoing (open, closed or temporarily closed to recruitment). Among active trials specifying at least one country as a site location, 909 are multinational, and as such, there will be instances where the same trial will overlap multiple countries. In typical fashion, the US is the most common playing ground by far with 1,890 trials. Destinations in East Asia and Western Europe follow, with Japan (735 trials), France (547), China (536) and Italy (510) rounding out the top five.
Various regulatory agencies have put forth guidelines or created working groups to facilitate and encourage use of genomic biomarkers in drug development, which may help fuel the ongoing and planned trial activity in these locations. (See sidebar, Global Guidelines.)
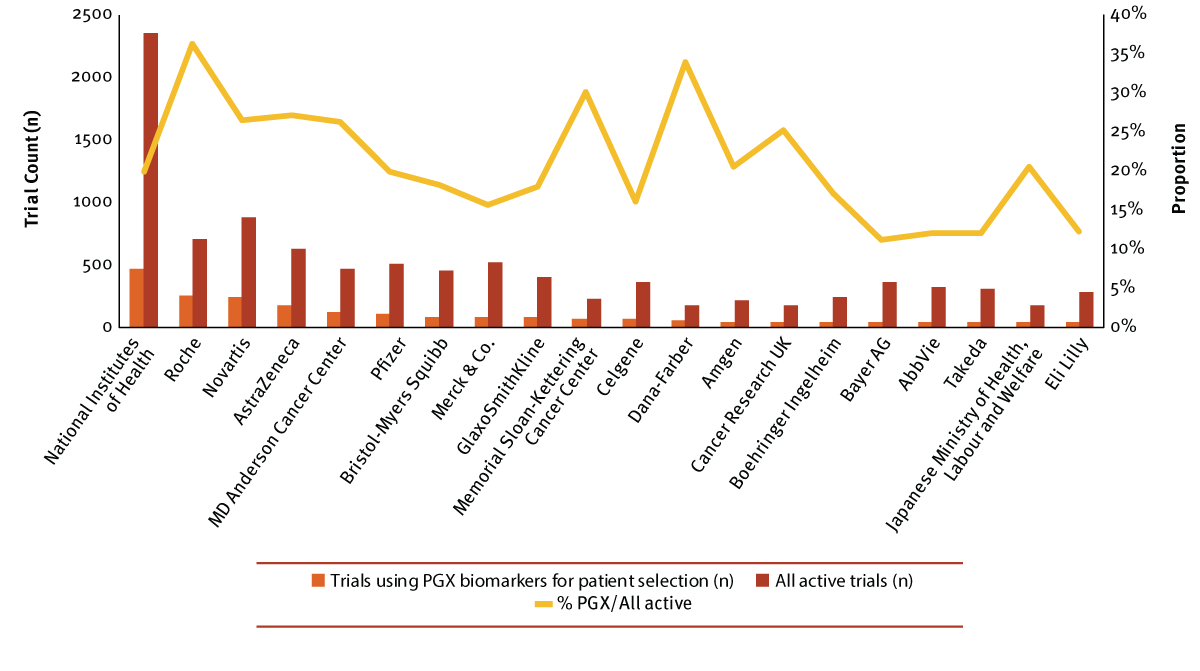
The roster of key players in the personalized medicine field consists of a mixture of government, industry and academic or medical centers. With the US as a leading location, it’s no surprise to see that the National Institutes of Health (NIH) is the most prolific sponsor and/or collaborator with 465 active studies using PGX biomarkers for patient selection, primarily driven by the NCI branch. However, personalized medicine is only one of many priorities for the government agency as it comprises 20% of all its currently active trials. Roche, Novartis AG and AstraZeneca PLC follow, which is in line with each company’s strong oncology focus and the frequency of oncology trials targeting specific patient populations. This approach appears to be a high priority for Roche – 36% of all its active clinical research leverages PGX patient selection biomarkers, which is the highest proportion among the top 20 sponsors/collaborators. The second highest proportion is held by the Dana-Farber Cancer Institute, an affiliate of Harvard Medical School and a Comprehensive Cancer Center designated by the NCI. (See Exhibit 4.)
Exhibit 4
Top 20 Sponsors/Collaborators Of Active Trials Using PGX Biomarkers
Note: Active trials are currently planned or ongoing (open, closed or temporarily closed to recruitment).
Trialtrove | Pharma Intelligence, 2017
Do Targeted Approaches Affect Drug Development Outcomes?
With the growing pursuit of personalized medicine and exploration of biomarkers to inform the appropriate treatment for patients, it’s worth reflecting on the past to determine whether this precision has an effect on the outcome of a drug development program, or the individual trial.
In an effort to measure clinical development success rates and strengthen benchmarking metrics for drug development, a prior analysis leveraged data from Informa Pharma Intelligence's Biomedtracker and Amplion Inc.’s BiomarkerBase to evaluate individual drug program phase transitions, defined as movement out of a clinical phase (i.e., advancing from Phase I to Phase II development), from January 1, 2008, to December 31, 2015. The analysis also considered a drug’s likelihood of approval (LOA), which represents the probability of reaching FDA approval from a current phase, calculated for a specified disease group based on the historical performance of drugs within the same disease group and development phase. Among these company-sponsored, FDA registration-enabling development programs, the use of a selection biomarker does appear to be beneficial, raising both the probability of success as well as the LOA. From Phase I to FDA approval, a three-fold higher LOA was calculated for programs that utilized selection biomarkers (25.9%) in comparison with programs that did not (8.4%). In other words, programs using selection biomarkers had a 1 in 4 LOA, whereas programs without selection biomarkers had less than a 1 in 10 LOA.
All four phase transition success rates were much higher for programs that incorporated selection biomarkers in comparison with those that did not. The largest percentage difference among the four phases of development was observed in Phase III transition success rates – Phase III programs using selection biomarkers had a rate of 76.5%, while programs without selection biomarkers had a rate of only 55.0%.
Considering that the use of selection biomarkers seemingly has a positive effect on a drug program’s ability to transition to the next phase as well as its likelihood of approval, we also examined the effect on individual clinical trials. The dataset included Phase I to IV completed trials sponsored by industry or large cooperative groups, comparing PGX biomarker selection trials with trials evaluating biomarkers only or no novel biomarkers, and the outcome analysis considered primary endpoints of the trial, classifying them into three categories: positive outcome/primary endpoint(s) met, negative outcome/primary endpoint(s) not met, and outcome indeterminate/unknown (categories of positive, negative and unknown in Exhibit 5, respectively).
Each trial category reported positive outcomes for over half its completed research. Although trials selecting patients by PGX biomarkers demonstrated the highest proportion of 55.3%, both non-biomarker and biomarker only studies were close behind with rates near 53%. Non-biomarker trials actually had the lowest proportion of trials reporting a negative outcome of 9.6%, versus 13.7% of PGX biomarker selection studies and 16.8% of biomarker only trials. Although non-biomarker trials were over five times more likely to have a positive outcome compared with a negative outcome, there does appear to be a slight advantage across biomarker research in using genomic markers for patient selection. Trials using PGX biomarkers for patient selection were four times as likely to report positive outcomes, while those using biomarkers for other purposes were three times as likely. (See Exhibit 5.)
Completed Trials
Exhibit 5 also provides a breakdown of the completed trials into oncology and non-oncology indications; proportions shift slightly across categories and outcome types, with oncology less likely to report positive outcomes in comparison with non-oncology. Regardless of the indication, there still appears to be a benefit among biomarker trials in incorporating selection markers – studies matching patients to therapy based on genomic markers continued to be around four times as likely to have a positive result, and biomarker only trials were still three times as likely.
One difference was observed in non-biomarker cancer trials. Across all therapeutic areas, non-biomarker trials were over five times as likely to have a positive result, which was still the case for these trials in non-oncology indications. However, this apparent edge decreases for oncology, and PGX selection trials without any biomarkers were only four times as likely to have a positive outcome.
A limitation of this analysis is the fact that it is prone to reporting bias, including delayed or incomplete disclosure of trial results. Over a third of trials included in the analysis were classified as ones with indeterminate/unknown outcome. A portion of these trials may well fall into the positive category, but potentially a number of these trials have negative results that have not been reported to date.
Exhibit 5
Effect On Completed Trial Outcomes Of Biomarker Use
*Indeterminate designation is given to trials that reported an outcome that was neither clearly positive nor clearly negative. Unknown is given to trials that have not yet reported full results for the primary endpoint(s).
Trialtrove | Pharma Intelligence, 2017
Terminated Trials
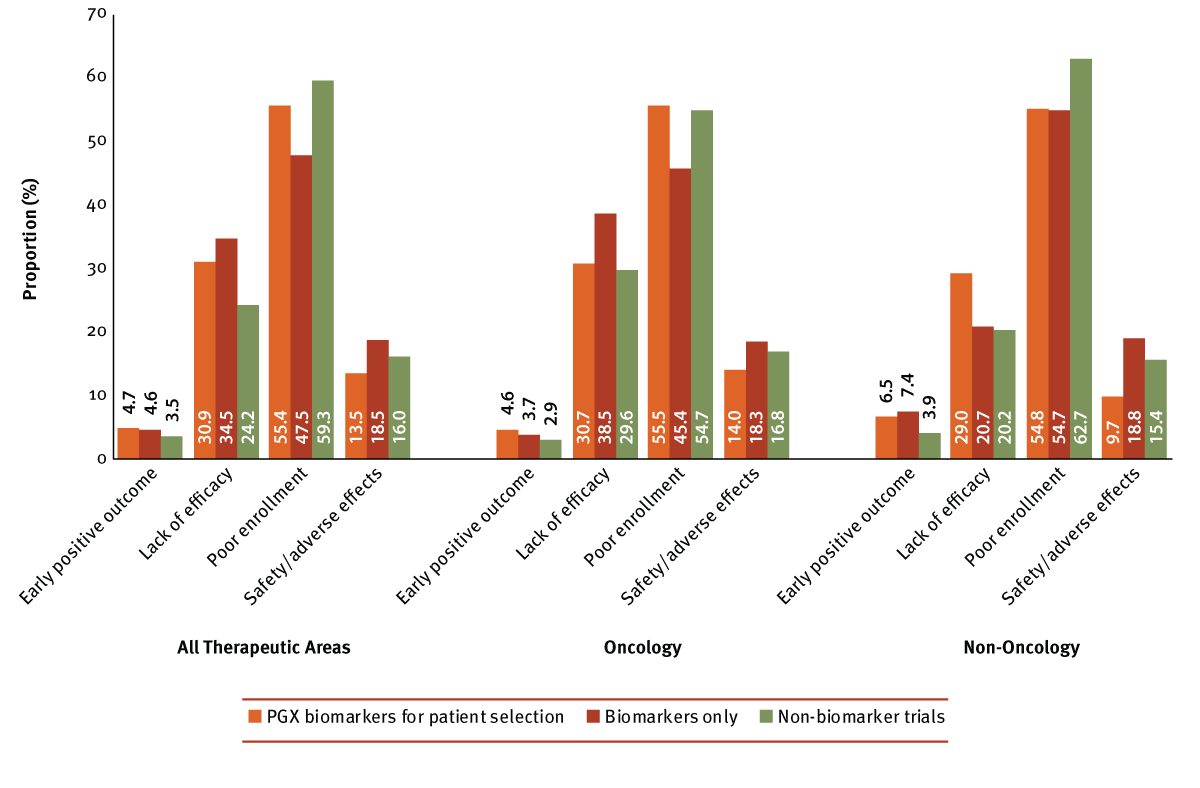
To further explore potential links between biomarkers and trial outcomes, we reviewed trials terminated due to reasons outside of strategic business decisions, specifically studies that prematurely ended based on early positive outcome, lack of efficacy, poor enrollment and safety/adverse effects. A total of 6,832 Phase I to IV trials have been terminated for at least one, or more, of these reasons as of March 2017, with poor enrollment as the most common reason for trial termination, speaking to the general challenges of patient recruitment. (See Exhibit 6.) Although a key concern for all trials, recruitment could pose a larger issue for studies selecting patients based on PGX markers due to the specialized populations involved for these trials. A higher proportion of PGX biomarker selection trials did indeed terminate due to poor enrollment compared with biomarker only trials, but at a lower rate than non-biomarker trials. As such, it appears that biomarker research in general is less prone to, but not exempt from, issues with recruitment rates, particularly for reasons outside of patient selection.
All three trial types had similar rates of trials terminating early due to an early positive outcome, which was generally a rare occurrence. The same is not true for the opposite side of the coin as non-biomarker trials were least likely to terminate due to lack of efficacy (24%), followed by PGX biomarker selection trials (31%). The advantage for targeted approaches, albeit slight, seems to lie with safety – trials with genomic markers were least likely to terminate due to safety/adverse effects.
Again, some differences are observed when limiting the dataset to oncology indications alone. A few proportions and trends remain largely unchanged; however, it appears that lack of efficacy becomes a larger issue for oncology trials without any biomarkers, and increases to nearly the same percentage as PGX biomarker selection cancer trials. In turn, the rate of non-biomarker cancer trials terminating due to poor enrollment decreases to a similar rate as studies with PGX driven patient selection. Shifts are more apparent with non-oncology indications, and PGX selection trials were most likely to terminate due to lack of efficacy. Also, issues with poor enrollment affected all biomarker trials equally, but they still terminated at a lower rate than non-oncology trials without biomarkers.
Exhibit 6
Effect On Terminated Trial Outcomes Of Biomarker Use
Trialtrove | Pharma Intelligence, 2017
Conclusions
While the traditional strategy of developing broad medical treatments for heterogenous patient populations has not been completely abandoned, personalized medicine approaches, and the acknowledgment that one-size-does-not-fit-all, are clearly being embraced across industry, government, and academic and cooperative groups. Luckily, the use of selection biomarkers to include or exclude patients into a trial does appear to have a positive effect on the success of the overall drug program, particularly for the drug’s likelihood of approval, although effects at the individual trial level are less clear. Considering the historically low rates of cancer drugs reaching the market, increased efforts to support this targeted approach for oncology should boost the odds of more effective options for more subsets of cancer patients in need.
Doro Shin ([email protected]) is Thought Leadership Program Manager for Informa Pharma Intelligence.