HCV Patient Access: Trek Therapeutics Takes The Road Less Traveled
Executive Summary
Trek Therapeutics, a three-year old biotech founded by a group of virology veterans, is testing the conventional wisdom in seeking to carve out a potential $17 billion business in the hotly contested hepatitis C space.
- Trek claims that projections of declining growth in the HCV segment seriously underestimate the impact of random diagnosis and chronic undertreatment; despite the arrival of a cure, HCV is far from a mature business.
- Trek's strategy depends on finding undiagnosed cases and then treating them with a curative standard of care that delivers value not only in the clinical setting but from a pricing standpoint as well.
- The company's contrarian vision is based on a balance between profit and public welfare. It’s a bet on a new model of organization, marketing and value assessment that could either fizzle or, alternatively, strike a chord among payers with an increasing public health orientation – demanding a standardized measure of efficacy for HCV at the lowest possible price.
- So what? Why should all this matter to the competitive drug landscape for infectious disease? It’s that old, primal threat of strategic disruption: a trend toward category commoditization along with the incremental innovation from a shorter treatment duration could leave the big pharma majors exposed to smaller, more efficient niche players able to meet payer desires for aggressive contracting at lower prices.
Biopharma has been talking for years about the necessity for a new business model, one that reconciles the aim of expanding patient access to the latest medicine at a price point conducive to supporting future innovations. Slowly, however, complacency is giving way to experimentation. Those big pharma imperatives of size, scale and reach mean fresh opportunities for niche players willing to operate at the margin – as overall industry sales build toward the trillion-dollar mark, can there be lucre in the leftovers?
In a first for biopharma, a team of industry veterans from the virology field has seized on the precedent of an iconic consumer brand – the ecology outfitter Patagonia Inc. – to create a start-up business dedicated to new treatments against infectious diseases, with an initial focus on a selective sliver of the estimated 135 million people worldwide with chronic, undiagnosed hepatitis C (HCV). The company, Trek Therapeutics PBC, launched in 2014 as a public benefit corporation, a variation of the standard legal approach to incorporation. A PBC permits a board to supplement the legal obligation to act in the financial interest of shareholders with a larger humanitarian, public welfare goal – in Trek’s case, making biopharmaceuticals for unmet medical needs that are affordable and accessible to patients. Some 33 US states allow for the PBC form of incorporation, which gives boards of directors legal protection when considering actions other than maximizing profit. There is a non-legally binding variation of the PBC called a “B-corp.,” but Trek opted for the stronger PBC label to prove its commitment to socially responsible drug access.
Dual Mission For One Disease: HCV
“It’s important for biopharma to examine precedents in adjacent industries with a strong pro-consumer orientation, which is how we came across Patagonia founder Yvon Chouinard,” Trek CEO Ann Kwong, PhD, tells In Vivo. Chouinard had several big shareholders complaining about his wasting money on research for projects like an environmentally friendly wet suit. The friction finally led to him persuade his board to adopt what was then a very new concept: registering as a public benefit corporation. “We like the fact that, years later, Patagonia retains a reputation among customers for responsible environmental behavior that translates to real enterprise value for shareholders. It’s a connection that’s still elusive in biopharma, which is precisely why we have embraced it,” says Kwong.
In fact, Trek is one of only two biopharma companies that have applied the public benefit model to date; the other is Perlara PBC, a California-based biotech focused on highly personalized treatments for rare disease patients with few options.
Despite its novel business structure, Trek is plotting a well-traveled path to therapeutic success: the HCV space now dominated by Gilead Sciences Inc., AbbVie Inc. and Merck & Co. Inc. The HCV connection was forged by Kwong herself, an industry veteran in infectious disease who became disillusioned with the cumbersome big pharma process after stints as a herpes virus specialist at Schering-Plough Corp.and as head of the antiviral division at Vertex Pharmaceuticals Inc., where she led development of Incivek (telaprevir), an HCV treatment tagged as the most successful drug launch in history until its withdrawal in 2014, after Gilead’s Sovaldi/Harvoni (sofosbuvir/ledipasvir) combination produced the definitive cure for the disease. “What frustrated me is how slow others in the industry were to recognize the global level of unmet need for HCV and address it proactively,” says Kwong. She cites as an example the failure of Vertex and its Asian licensee, Mitsubishi Tanabe Pharma Corp., to launch Incivek to a projected treatment population of 10 million in China on grounds that the prospective price was too low.
There is a sound – and profitable – commercial rationale for action by companies able to offer these treatments at a price that will increase access to neglected populations that, for a variety of reasons, remain outside the orbit of the big pharma majors.
Trek’s business proposition consists of two simple points. First, private-sector research has brought great new medicines to the market, with the potential to extend from HCV to other major infectious diseases. Second, there is a sound – and profitable – commercial rationale for action by companies able to offer these treatments at a price that will increase access to neglected populations that, for a variety of reasons, remain outside the orbit of the big pharma majors.
According to Kwong, this is no small change. “We estimate the 10-year global market value of the untreated population with chronic HCV is upward of $17 billion. What I find from my visits with most US investors is they look at Gilead, see its revenues coming down and conclude that the HCV market is done. Yet the demographics show that HCV remains a vastly undiagnosed condition – even here in the US – and will remain so as long as the price for the cure is high. What we have now is a $140 billion protected market controlled by the three leading drug producers, who prefer price to volume as a revenue driver.”
In contrast, Trek is focusing on driving volume to a larger number of patients who occupy a small sub-set of the overall market. Big pharma players’ interest remains centered on patients in the rapidly shrinking 25% of the US and European markets that is already diagnosed. Even though the number of patients is smaller, the premium prices charged to these markets makes them far more profitable for the companies.
The V-Team
Recent surveys on the extent of the undiagnosed HCV population bear out Kwong’s assertion, underscoring the rationale for a new business opportunity. To tap the potential from the undiagnosed, Kwong has assembled a team of industry veterans to move the start-up forward. All have a background in antiviral and infectious disease research from companies like Pharmasset Inc., Gilead Sciences, Vertex, Genzyme Corp., Roche and Bristol-Myers Squibb Co. Trek’s co-founder and current board chair, Jerry Zeldis, MD, PhD, hails from Celgene Corp. (also an equity holder) where he was responsible for the launch of blockbusters such as Revlimid (lenalidomide), Otezla (apremilast) and Abraxane (paclitaxel protein-bound).
Their skills are crucial to Trek’s goal of building a commercial platform to deliver its first marketed HCV drug in Europe and the US by 2021. Access to therapy will be based on a negotiated, contracted price model targeting selected underserved HCV populations, initially in the US, Europe and Mexico. Kwong calls it the “test and treat” strategy, with the emphasis on finding undiagnosed cases and then treating them with a curative standard of care that also delivers value, not only in the clinical setting but from a pricing standpoint as well. In fact, the Trek model is founded on the idea that the high prices for HCV cures is the single most important barrier to increased diagnosis of the disease.
The Trek model is founded on the idea that the high prices for HCV cures is the single most important barrier to increased diagnosis of the disease.
The criteria for supply is relatively straightforward. In high-income countries like the US, where the current standard of care relies on HCV cures accessed at net wholesale prices from $40,000 to $95,000 per course of treatment, Trek will focus on the prison population, the Indian Health Service and other “price sensitive” constituencies. These require close attention to fiscal budgetary shifts as well as the cultivation of close ties to local political communities. Obviously, pricing is critical and volume-based contracting is becoming the norm.
In middle-income countries, including central and eastern Europe as well as Mexico, the targets are undiagnosed patients where pricing of curative drugs, at least through 2015, varied from $15,000 to $95,000. The predominant standard of care consisted of a previous generation of treatments like the peginterferon alfa/ribavirin combination, which are hard for patients to tolerate and carry significant side-effects. Currently, their access to current standard of care oral cures is limited to slots in special registries set up by government health authorities, which prioritize who gets treatment first, based on available resources. The remaining patients are forced to pay out-of-pocket the list price or simply progress in their disease while waiting for a treatment slot.
To expand its reach in making its own drug regimens available to the undiagnosed in the biggest middle-income markets – notably China and Russia – Trek anticipates working with local partners on an out-licensing basis.
In the US, Trek has developed a specific platform it calls INSPIRE (Insurer and Pharma Initiative to Create Affordable and Accessible HCV Treatment). INSPIRE is intended to structure discussions with all kinds of insurers on payment for medical care, including commercial health insurers, public health authorities, ministries of health, prison systems and other institutions. The goal is consensus with these stakeholders around financing for coverage of Trek’s TRK-1sd six-week HCV regimen, when it hits the market as scheduled in 2022. The preferred tool is the advance purchase agreement, a concept introduced a decade ago under sponsorship of the G-7 governments to support vaccines for neglected diseases.
The benefit for Trek is advance purchase will support late-stage development work on TRK-1sd, provide a predictable revenue stream going forward, and increase access through coverage for undiagnosed HCV patients. The expectation is it will also help draw in other insurers to increase access for the Trek HCV line-up across the country, extending the potential market beyond the originally envisioned targets such as the federal/state prison systems and the Indian Health Service. Kwong also tells In Vivo the INSPIRE model is being promoted to middle-income countries outside the US and Europe.
Trek VP for corporate and medical affairs Cami Graham, MD, expresses excitement at talks Trek has been having with the World Health Organization (WHO) on how to introduce a low-cost diagnostic for HCV that would be effective in raising access to cures in all markets, rich and poor. “We plan to work with different diagnostic companies and coordinate with payers or pay for diagnostics by ourselves to conduct intensive test and treat campaigns. Patients then diagnosed with HCV infection can choose to be treated with a Trek regimen or one of the other standards of care marketed by other companies.”
While the emphasis for now remains on establishing a profitable enterprise by capturing undiagnosed, dependent populations in rich and middle-income markets, there are many opportunities beyond that. Says Kwong: “One of the things we talk about is, once we have an ongoing business, to create an in-house VC fund to create new PBC access strategies, not just for HCV but for other infectious, high social burden conditions as well. Giving back to patients has to be ingrained in how biopharma conducts itself as a business – there is no separating the two.”
The Double Aim: Affordable Access
Assuming its HCV pipeline of next-wave cures pans out, Trek anticipates launch at a price point of just under $10,000 per treatment course. The company believes this will open a target patient base of 55 million people – 10 million in the US and the big five European nations and another 45 million in middle-income countries (i.e., eastern Europe and Mexico) – or about 40% of the worldwide estimate of undiagnosed HCV cases.
Assuming its HCV pipeline of next-wave cures pans out, Trek anticipates launch at a price point of just under $10,000 per treatment course.
The remaining 60% are among the poorest victims of HCV in low-income countries, a cohort that has no role in Trek’s business plan. Says Kwong, "For the 101 countries that theoretically have access to generic version regimens through voluntary licenses with local manufacturers in India, Pakistan and Egypt, the drug price is $250 to $900 per course of treatment. Trek cannot manufacture our drugs at a cost equal to or lower than that. So these countries do not need the solution we can offer. But we applaud Gilead and BMS for making their curative products available at generic prices in these countries.”
Big Pharma: How Low Will It Go?
Kwong is confident that, as a start-up, Trek has the flexibility to exploit opportunities where the big players like Gilead cannot. Here’s the pregnant question: Why wouldn’t Gilead just cut its $45,000 price for Sovaldi/Harvoni (after rebates and discounts) to $10,000 to undercut Trek’s pricing proposition to payers and broaden its market base in central and eastern Europe?
In response, Kwong contends that would force Gilead to reduce its 10-year revenue projections in Europe by one-half while taking on treatment responsibilities for nearly one million new undiagnosed patients, which also increases the likelihood of price leakages to local customers with the propensity to pay more. “A ROI model structured around patients already diagnosed with HCV means Gilead has an incentive to restrict the number of treatments allowed at a given price point. The company conducts extensive monitoring to make sure its generic HCV version doesn’t leak from very low-income and high-incidence countries like Egypt to the premium-priced markets. Gilead also supports outreach to manage HCV progression to cure, which imposes extra costs in administration of therapy. And reliance on current estimates of the diagnosed population gives you a finite number on market potential, which helps explain why Gilead’s revenues from HCV are decreasing. Taken together, these factors have convinced us the big players aren’t going to steal the undiagnosed and underserved disease cohort away from us.”
In fact, Kwong believes that third-generation combination products now moving through early-stage trials will accentuate the majors’ need to keep prices high. (See Exhibit 1 for Datamonitor Healthcare's list of HCV drugs in early-stage development.) “Because of the high cost of treatment, nothing will change in the motivation US payers and insurers have to keep demanding four-week refill authorizations in the course of the standard 12-week treatment cycle. The feedback we have from payers is that if we can offer a six-week cycle at $10,000 per patient, then prescribing physicians may only have to request it once – or maybe not at all. That’s a big commercial advantage.”
Exhibit 1
Select HCV Drugs In Early-Stage Development
Datamonitor Healthcare | Pharma Intelligence, 2017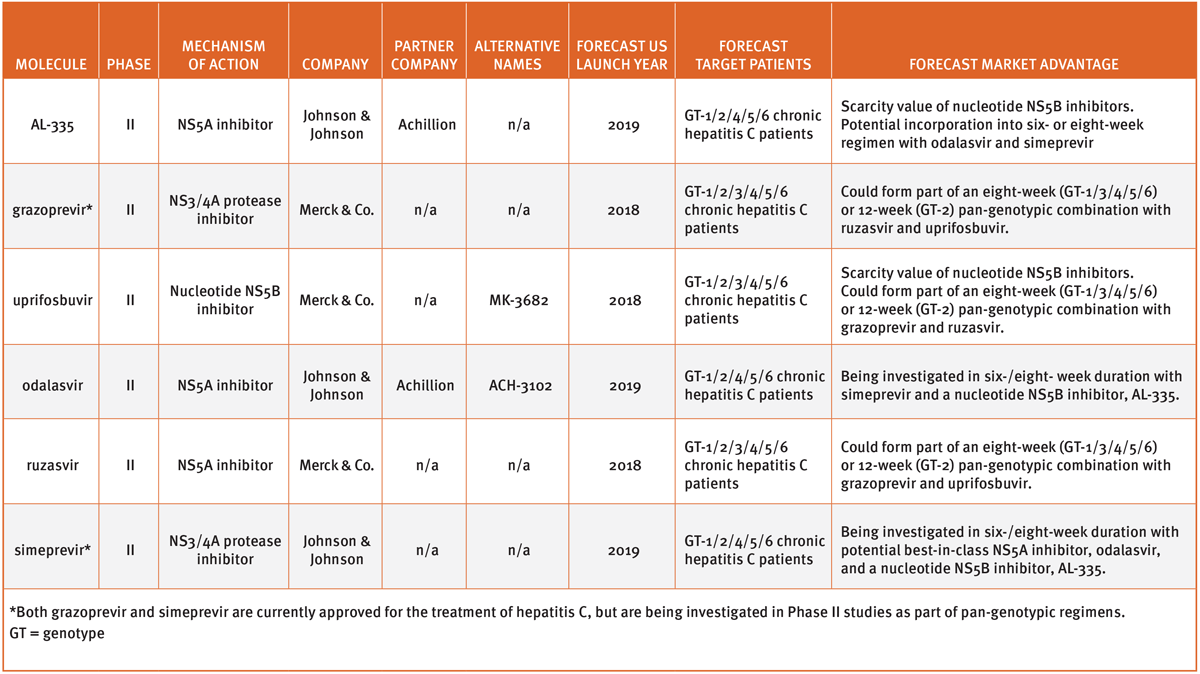
Five Compounds: In Five years?
Identifying sales potential means little without a productive R&D portfolio, so how close is Trek to a marketable drug? Currently, Trek’s development program consists of five compounds, all acquired from other companies and indicated for HCV and hopefully other infectious diseases in the future. Two of the compounds (VX-222, a first-generation NNI; and VX-497, a broad spectrum antiviral IMPDH inhibitor) were acquired from Vertex. [See Deal]
Rights to the other three (Faldaprevir [FDV], a second-generation HCV protease inhibitor; TD-T6450, a third-generation HCV 5a inhibitor; and MIV-802, a third-generation HCV nucleoside) were secured from Boehringer Ingelheim GMBH, Theravance Biopharma Inc., which holds an equity stake in Trek, and Medivir AB, respectively. [See Deal][See Deal]
There is an out-of-the box advantage here, as each of the four other products had undergone some form of testing under the previous asset holder: Faldaprevir, the protease inhibitor acquired from Boehringer-Ingelheim, had completed and submitted NDA’s to the FDA and the EMA when the company abandoned the HCV space after a 2014 portfolio review. “We got the whole package,” Kwong says.
Trek's HCV Portfolio
Faldaprevir: HCV protease inhibitor acquired from Boehringer Ingelheim
TD-6450: HCV NS5A inhibitor acquired from Theravance Biopharma
VX-222: Non-nucleoside inhibitor of the HCV polymerase acquired from Vertex Pharmaceuticals
VX-497: IMPDH inhibitor acquired from Vertex Pharmaceuticals
MIV-802: Nucleotide inhibitor of HCV polymerase acquired from Medivir AB
SOURCE: Trek Therapeutics
Trek’s clinical focus is on slashing the treatment duration for HCV, from the current 12-week standard of care to six weeks. Trek is also testing its acquired compounds selectively with older therapies for HCV like ribavirin, previously used in combination with interferon. The aims are to define the best drug combinations to increase drug potency, to reduce duration of treatment, and to characterize HCV genotype coverage. Another objective is to refine the chemistry of HCV drug development to improve safety, quality and manufacturing efficiency and, ideally, produce a fixed-dose combination drug product. As noted, Trek is positioning these formulations for potential out-licensing partnerships in large emerging country markets such as Russia and China, where local ties are often a precondition for access.
A lot of rubber has to burn for the company to meet its aggressive 2021 launch date for the first HCV drug, TRK-1, a combination of FDV, the TD-6450 5a inhibitor and a non-nucleoside inhibitor. It is indicated for the most common genotype one and four strains of HCV. Now in clinical study Phase IIa, it is being positioned against AbbVie’s Viekira Pak, the second biggest selling HCV treatment after Harvoni. MIV-802, a drug that will be used in a second regimen, is undergoing animal and toxicity testing and will enter Phase I proof-of-concept studies soon. A second, short duration drug, TRK-1sd, a fast follower variation of TRK-1, also targets HCV genotype one and four. It has the potential to be positioned as a six-week drug against the 12-week regimen shared by all the current leading curative therapies – a major “best in class” improvement in standard of care. TRK-1sd is slated for launch in 2022.
A third HCV drug, TRK-2/3, is indicated for genotype two and three strains of the disease. A combination of the Medivir nucleoside drug MIV-802 with one or two other drugs, it is being developed for an eight-week to 12-week treatment duration and a launch in 2023.
Investors: Will They Take The Long Bet On Trek?
Trek has much distance to cover before formal trial data can be applied to speed the registration process. Pilot Phase IIa studies to evaluate safety and efficacy issues like dose response are about to start for TRK-1 and in 2019 and 2020 for TRK-1sd and TRK-2/3, respectively. It’s a tight schedule, with the company projecting finishing the Phase III work on TR-1 only a few months before the 2021 year-end target for launch of its first marketed therapy.
Transitioning to trials with a larger base of human subjects is going to require additional resources. Trek continues to rely on a $10 million Series A equity float obtained from investors when the company was founded in 2014. Management is now pitching to investors a Series B financing package totaling $45 million, to complete Phase IIa and prep for Phase IIb studies. Kwong and team are concentrating on high-net-worth “family office” investors in Europe, many of whom are seen to be philosophically in tune with Trek’s PBC model. Earlier this year, with support from its lead investment banker, New Century Capital Partners, Trek presented its business plan to 62 investors in five European cities.
Kwong avows that the focus on European money is deliberate, as health care is widely considered a basic human right in Europe. Management suggests that European payers will be more likely to embrace the Trek model than payers in the US. “Actually, we anticipate our first launch of TRK-1 will take place in Europe.” Kwong tells In Vivo the plan is for Trek to set up a holding company based in the EU once it obtains its $45 million funding target, and then proceed to a European IPO during Phase II clinical studies, with a dual listing on the Nasdaq or its smaller European equivalent, Euronext Growth, to fund Phase III and commercial launch of TRK-1.
Nevertheless, could that fallow period between the business plan and a saleable drug end up being too long for investors to claim the harvest? Some investment analysts think so. “Even in the aftermath of a cure, there remains additional room for innovation in HCV therapy,” says Les Funtleyder of E Squared Asset Management. “We should see more activity among the big companies in HCV with drugs that reduce the duration of treatment while minimizing the possibility for relapse. Along with more incremental improvements in tolerability, these are likely to spark reductions in pricing.” In other words, by the turn of the decade experts are predicting price competition will be the dominant characteristic of the HCV segment.
Counting On Disease Awareness
If its borne out, this new market dynamic will test what Trek claims as its key competitive advantage: pricing that the bigger players won’t – or can’t – match. There are already examples where the industry has agreed to negotiate deals with governments to slash prices in return for guaranteed access to large patient populations – a five-year price/volume agreement signed in 2016 with the Australia health authorities is a case in point.
Still, Kwong contends that the sheer size of the untreated population is sufficient to give small, efficient players like Trek a good slice of the market, assuming its drug portfolio keeps pace with the advances in science. Graham and others cite the work of economist Homie Razavi, PhD, of the Colorado-based Center for Disease Analysis, in documenting how far the world has to go in bridging the gap between HCV prevalence and treatment. (See sidebar, “Start-Up's Stand-Out Stance on Price.”) Estimates published this year by his group indicate that the new curative drug therapies are slow on the take-up: 94% of those receiving the cures were located in only six countries – the US, Egypt, Spain, Germany, France and Pakistan.
At this rate, the Center says, it will take till the end of the century to find, treat and cure everyone with HCV. Adds Graham, “The WHO has declared a goal of eliminating HCV globally by 2030. We see the WHO commitment as one that will lead to stepped-up pressure to identify and treat the undiagnosed after 2020, which conforms nicely to our time line of entering the market with a flexible, accessible and low-cost pricing structure that should appeal to new categories of payers.”
Time – and the willingness of more HCV patients to search, speak out and step forward – will tell if Trek has found the right path to the market.
