New Payment And Financing Models For Curative Regenerative Medicines
Executive Summary
Cell and gene therapies that have the potential to cure require new approaches to value assessment, payment and financing. In the second of a series, the Alliance for Regenerative Medicine identifies potential payment models, highlights key stakeholder concerns and identifies the barriers that must be addressed to enable their integration across the health care system.
- Traditional payment and financing models may not be appropriate for curative treatments, some of which may require only a single administration.
- It's imperative that new payment and pricing models balance patient access and affordability but also adequately value curative therapies.
- There is no “one size fits all.” The variety of technologies and approaches means that multiple options may be needed, and in some cases will need to be combined.
- So what? A number of proposed reimbursement and financing models are available to address the potential uncertainty and economic disincentives that may be associated with curative therapies. Implementing them will be critical to ensuring patient access to those medicines and continued innovation in the field.
The next decade promises a wave of innovative and potentially curative gene and cell therapy products representing breakthroughs for patients with devastating and costly diseases, many of which lack current treatments. Patients with certain forms of inherited blindness, rare genetic muscle and neurological disorders, hemophilia, untreatable cancers and other serious conditions may soon have transformative treatment options for the first time in the form of gene therapies, gene editing technologies and cell therapies such as CAR-Ts (chimeric antigen receptor T cells), and other types of regenerative medicines. As discussed in our previous article, many of these therapies are expected to provide durable and profound treatment effects with a single administration of therapy, effectively curing the disease or condition. (Also see "Curative Regenerative Medicines: Preparing Health Care Systems For The Coming Wave" - In Vivo, 15 Nov, 2016.) However, traditional payment and financing models based on cost-per-unit of product or per procedure may be suboptimal to support adoption, patient access and continued innovation of these therapies.
These therapies are expected to deliver their benefits over a long period of time, ranging from several years to the remainder of a patient’s lifetime. However, under the current payment paradigm, the costs of many of these therapies will by necessity be incurred at the time of administration, as has occurred with the initial launches of in vivo and ex vivo gene therapy in Europe (uniQure NV's Glybera and GlaxoSmithKline PLC's Strimvelis, respectively).
Although these therapies offer the potential for improved health, quality of life, productivity and reduced costs, some stakeholders are concerned. For example, under routine payer therapeutic management techniques and existing benefit designs, patients worry access to life-saving medications may be unaffordable given anticipated high out-of-pocket costs. In addition, payers and providers have expressed concern that they could pay high up-front costs for therapies that will not yet have evidence to support the projected long-term benefits and for which the value cannot yet be fully characterized. Budget impact is also of concern to payers and providers, especially for therapies to treat a sizeable patient population.
In addition to ensuring patient access to new therapies, innovator companies, which invest substantial resources into the development, manufacture and launch of these therapies, are concerned that reimbursement systems will not fully account for the value these therapies provide.
All stakeholders (including patients, providers, payers, etc.) need new models of payment and financing for gene and cell therapies that address these key concerns. There is no “one size fits all” and the variety of technologies and approaches means that multiple options may be needed, and in some cases would need to be combined. The system needs to be flexible enough to accommodate different models. This article, the second in a series of three, will further describe the key payment and financing models that have been identified to date, examine their ability to address the concerns of various stakeholders, and identify the necessary steps to address barriers to implementation.
Alternative Payment And Financing Models
In most health care systems, the costs of curative therapies and the procedures necessary to enable their administration will be incurred and payable at the time of each administration, based on the amount of drug and/or the procedures needed to enable the therapy. In some cases, this “Up-Front” or “One-Time” payment model might remain acceptable for the payment for certain low-volume, or well-characterized curative therapies. However, additional models have been proposed to address key stakeholder concerns regarding costs and uncertainty of outcomes should these therapies be indicated more broadly.
Several financing and payment models have been proposed to date as alternatives to one-time, up-front payment for single administration gene and cell therapies. (See Exhibits 1 and 2.) In Exhibit 1, payment models refer to how the product is paid for, for example, the transactional flow of funds to provide payment for the product and/or service, and Exhibit 2 refers to the source of funding, for example, who pays for the therapy.
It is possible that some models could be combined. For example, pay-for-performance arrangements could be combined with a one-time payment or annuity/installment payment. Additionally, an annuity/installment payment obligation taken on by a payer or institution could be passed through to a re-insurer. Risk pools could pay for gene and cell therapies via a periodic payment and/or pay-for-performance model, with patient assistance programs limiting patient out-of-pocket cost exposure.
Exhibit 1
Payment Models for Novel, Curative Gene and Cell Therapy Products
ARM
Exhibit 2
Financing Models for Novel, Curative Gene and Cell Therapy Products
ARM
Payment And Financing Models – Key Considerations
Each payment and financing model has attributes that address the needs and desires of some stakeholders better than others. Patients and their caregivers want their cost and risk exposure minimized, especially in the case of co-payments and co-insurance in multi-payer systems, where the burden on the patient could be substantial if new methods of offsetting patient cost-burden are not introduced. Payers are concerned with the uncertainty of the long-term outcomes and the potential budget impact of potentially curative new therapies. Although an innovator may prefer to have the full value of the therapy recognized and compensated at the time of administration, payers, providers and patients may desire that the costs of a new therapy be spread over time like chronic therapies and, therefore, aligned with the delivery of health benefit.
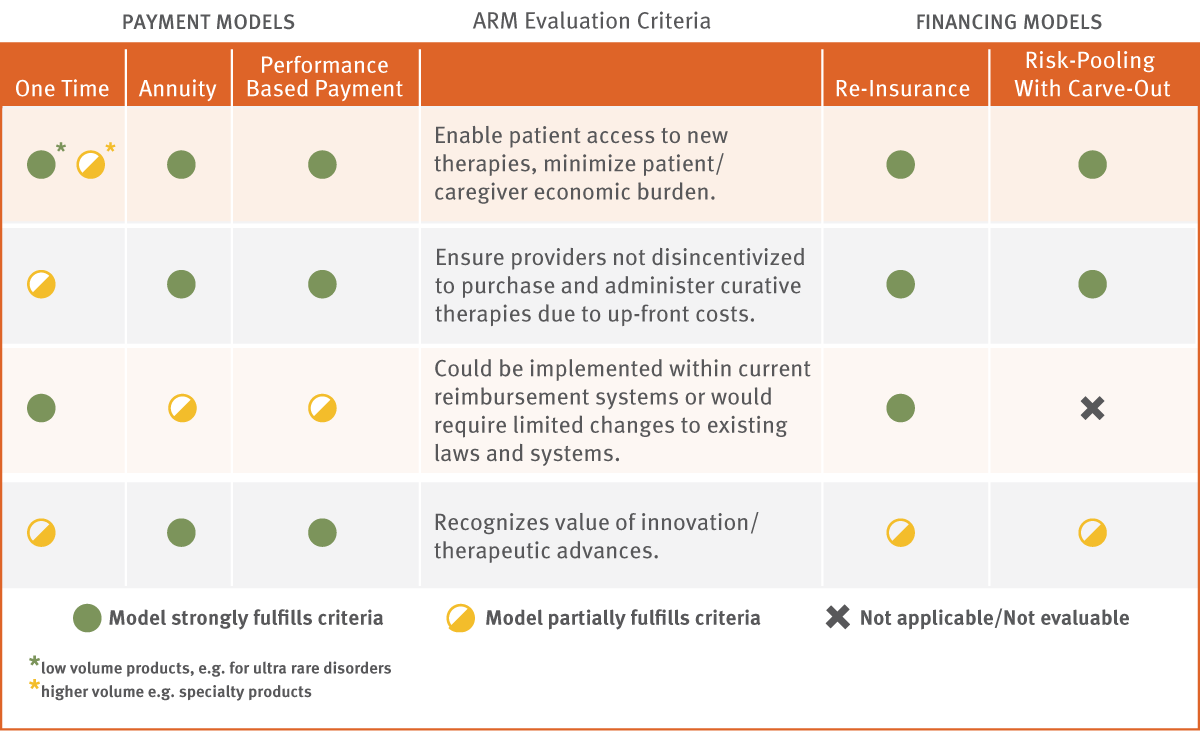
Payment and pricing models that adequately balance access and affordability but also sufficiently value curative therapies should be prioritized. We propose the following criteria for evaluation and prioritization.
Preferred Models Should:
- Enable patient access to new therapies and minimize patient/caregiver economic burden. Gene and cell therapies typically target patients with serious, sometimes life-threatening, health conditions with significant health burden, morbidity and poor quality of life. Models that place undue economic burden on patients and caregivers, by asking them to take on more than a reasonable co-payment or co-insurance burden, should be lower priority than other models.
- Ensure providers and institutions are not dis-incented to administer curative therapies due to up-front cost. Health care providers and facilities play a critical role in the introduction and adoption of innovative new therapies. Reimbursement and financing structures should not place an undue burden on providers and facilities with respect to working capital outlays at time of treatment, financial risk, or risk of outcomes for using new transformative therapies.
- Be feasible to implement in a time frame to promote product adoption. Changes in access, coding and payment policies and systems may be required to enable new payment and financing models in order not to impede patient access to innovative new gene and cell therapies. Solutions that could be implemented within current public and private payer systems, or that could be implemented within a short period with limited changes or clarifications to coverage policies, should be prioritized over more complex solutions. However, superior long-term solutions should be pursued even if they require significant changes to current systems.
- Recognize the value of innovation/therapeutic advances. Innovation typically requires private sector investment of hundreds of millions of dollars and several years of discovery and development, sometimes a decade or more from concept to product. The value of this innovation is the development of products that may treat or even cure currently unmet medical needs. In addition, the production of novel gene and cell therapies based on biological materials, sometimes utilizing the patient’s own cells as a starting point to develop an individualized therapy, can be far more complex and more costly compared with manufacturing traditional biological and small-molecule pharmaceutical products. Payment models must appropriately include the value of these therapies.
Below is a discussion of various payment and financing models and their application to cell and gene therapies.
Payment Models
Annuity Payment Models
Annuity payment models, also known as “periodic,” “amortization” or “leasing” models strongly fit most of the criteria for preferred models. Under these models, payments are made over time, thereby reducing large up-front costs. The benefits of these models includes the potential to reward innovation and to better align costs with the time period over which benefits are delivered to the patient, thereby reducing up-front budget impact to the payer or provider and reducing initial cost as a barrier to appropriate access for treatment-eligible patients.
However, implementation of a periodic payment arrangement for a one-time administered therapy is not without barriers. These include the seller reaching agreement on payment amount (net present value of payments) and duration with the purchaser, whether that is the provider/institution that administers the therapy and/or the payer. Payer accounting rules will likely need to change to comply with these payment arrangements.
Hospitals administering high-cost therapies could agree to payment terms with the drug manufacturer, and have a separate arrangement with the payer for appropriate payment to the health care provider. This would eliminate the requirement for the health care provider or institution to purchase the therapy at full, one-time cost and to bill the payer at full, one-time cost.
In addition, for patients with private insurance, the tendency of patients to switch plans (or become Medicare eligible) could mean that a patient is covered by one insurer with the costs of treatment continuing to be paid for by a prior payer, unless a mechanism is developed for the payments to become portable with the patient. Other barriers include the potential impacts on statutorily mandated discounts.
A possible solution to these problems would be to divide the initial costs of the treatment up into smaller payments payable over time, perhaps without tying the payment to performance, survival or continued coverage by the patient’s original plan. Also, an ongoing financial obligation could be made part of the patient’s pre-existing condition or covered under essential health benefits, to ensure continuity of coverage, as required by federal and state mandated benefits. If many payers engage in these types of arrangements, the flow of patients, costs and benefits across plans should eventually balance out. In addition, mechanisms would need to be created to allow for ongoing patient-assistance if there is an annual co-payment or co-insurance alongside annuity payments in the years following the one-time administration of a curative therapy. Medicare and Medicaid rules would likely need to be changed as well to accommodate new payment models
Pay-For-Performance Based Models
Performance-based risk-sharing agreements pay companies based on agreed-upon performance of clinical outcomes. These models are likely to be more easily implemented by payers – such as Medicare and Medicaid – whose enrollees stay with the plan over longer periods of time. Depending on how they are implemented and under what financial terms, performance-based models strongly satisfy several of the key evaluation and prioritization criteria outlined above.
These arrangements may be attractive for innovators in cases where evidence/predictors of durable effect are apparent in the short-term (several months to a couple of years) and may have already been characterized in clinical trials. Several key challenges have been highlighted in the previous section, including innovator and payer/or provider agreement on the definitions of product performance, product value under uncertainty, and payment amount and schedule as well as potential impacts on statutory state discount programs if implemented in the US.
Many products are likely to have data registries that patients will be strongly encouraged to participate in for post-treatment follow-up beyond post-marketing regulatory requirements, providing a means of data collection on product performance in aggregate and for individual patients that, with appropriate permissions and data safeguards, could be shared with all public and private payers in performance-based arrangements.
Financing Models
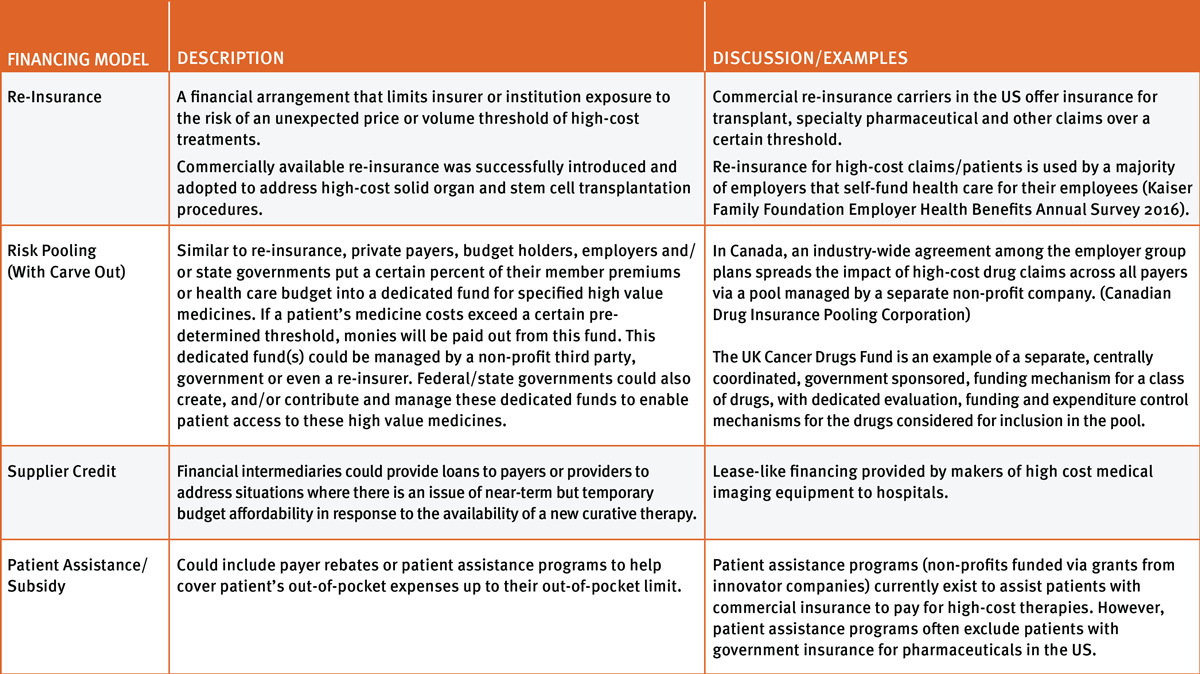
Re-Insurance
Re-insurance mechanisms could be adapted to curative therapies with limited need for change to coverage, coding or payment mechanisms. (See Exhibit 2.) In the absence of any changes in payment or finance models, re-insurance could be used with an up-front, one-time payment model to help offset any near-term budget impact for high-cost curative therapies. Re-insurance could also be combined with a periodic or performance-based payment model, with the re-insurer taking on the payment obligation and benefiting from any rebates or refunds should a patient fail a therapy.
Re-insurance mechanisms can be considered favorably in terms of limiting economic burden on the patient, ability to be implemented in a time frame that would not hinder product adoption, and ensuring providers and institutions are not t disincentivized to administer curative therapies due to cost. Concerns include the possibility of increases in the cost or reduced availability of re-insurance due to a new market, and more frequent and higher claims costs due to curative gene and cell therapies.
Risk-Pooling (With Carve-Out)
Risk-pooling mechanisms could work when there is stability in the health system, and transparency and alignment of interests of key stakeholders. Overall health system budget constraints and uncertainty regarding changes to the Affordable Care Act could remain an impediment to creating and funding such a pool in the US for patients appropriate for curative gene and cell therapies. Alignment on inclusion criteria for what product classes and products should be included in the fund, the cost threshold that triggers the payments and the valuation and payment for those products would need to be determined and aligned among key stakeholders. Risk-pooling focused on product classes or disease areas would need to be structured in such a way as to not place an undue incremental financial burden on patients in the form of excessive co-payments, lifetime coverage caps or increased future health premiums due to the presence of a preexisting condition.
The advantage of this model is that it offers budget predictability to payers/budget holders, prevents adverse selection, and no payer, including an employer, is adversely affected by having clusters of patients in their plan that require high-value cell and gene therapies. In addition, this method can be combined with various payment models (up-front with or without performance-based money-back guarantee or periodic payment with or without performance guarantee). This model allows individual payer autonomy on coverage decisions while decreasing financial risk.
Supplier Credit
The supplier credit model could come in the form of a third party that would act as a financial intermediary between manufacturers and payers or providers. The therapies would be sold directly to the third party (either a private entity or a fund set up by the federal government), then payment terms would be negotiated between the third party and the payer or provider.
This option could potentially work in conjunction with the annuity model to lower the risk of non-payment to the manufacturer and to help ensure payment is made when the patient changes insurance providers.
Patient Assistance/Subsidy
Patient assistance programs and subsidies could potentially help cover patients' out-of pocket expenses and reduce cost-burden to patients and their families. These programs are well established in the US, with a focus on providing co-payment assistance or free drug for low-income patients with commercial insurance. While these mechanisms would not be unique to curative gene and cell therapies, adaptations or modifications of these programs, when used in combination with the models outlined previously, could be part of a solution that mitigates affordability issues for certain patients. Challenges include adapting current programs to provide continued co-payment or co-insurance assistance to patients if an annuity payment model requires annual co-payments or co-insurance from the patient for continued payment by the payer.
Alliance for Regenerative Medicines
Conclusions and Recommendations – Where Do We Go From Here?
There are several potentially viable models for addressing the unique challenges of payment and financing for innovative, curative gene and cell therapies, each with its own advantages and disadvantages, and challenges in implementation. If multiple options are enabled and adopted, such flexibility could serve the needs of a number of stakeholders and allow for payment and financing models to be selected and adapted to the particular application. Product characteristics, payer needs, current reimbursement mechanisms and innovator needs may vary case-by-case. Exhibit 3 highlights those models that we view as higher priority based on our evaluation using the criteria outlined previously. The last article in our series will further define the legislative and logistics barriers to implementation of these models and will propose more detailed approaches to overcoming these barriers.
Ted Slocomb ([email protected]) is VP, Commercial Planning, Audentes Therapeutics; Michael Werner ([email protected]) is Co-Founder and Executive Director, Alliance for Regenerative Medicine; Ted Haack is VP, LatticePoint Consulting; Satish Valluri is Director of US Patient Access, Pfizer; and Beth Rader is Director, Market Access Strategy and Payer Policy, BioMarin. The authors gratefully acknowledge the contributions of Donald Han, Pfizer; Eric Strati, Mesoblast; Azideh Golipour, Avrobio; and Lyndsey Scull, ARM.