Thirty-five Years Covering Health Care: The More Things Change…
Executive Summary
The health care industry has come a long way in the past 35 years, although in some areas very little has changed. Recently retired In Vivo editor Peter Charlish has seen most of the major developments, and in his final feature, he looks back at some of the big stories in a reporting career that began in the early 1980s.
The health care industry has witnessed many changes in the past 35 years or so since I began reporting on it, yet in some ways very little seems to have changed. While there has been enormous progress in the treatment of many diseases, some of the challenges we faced back in the early 1980s remain today.
Perhaps it would be more accurate to say that problems previously thought to have been solved have come back to haunt us. One of the first stories I covered as science editor of Scrip in 1981 was the launch of Augmentin (amoxicillin + clavulanic acid), the antibacterial product developed by the UK company Beecham (now swallowed up into GlaxoSmithKline PLC), which was designed to overcome the problem of bacterial resistance.
The rationale behind Augmentin was elegant in its simplicity. Bacterial resistance to β-lactam antibiotics such as amoxicillin is often caused by the bacteria acquiring the ability to produce an enzyme, β-lactamase, that can break down the antibiotic and thus render it ineffective. By combining an inhibitor of β-lactamase with the antibiotic in a single product, this route to bacterial resistance is blocked. The enzyme inhibitor in Augmentin is clavulanic acid, a compound that is based on the same β-lactam ring structure as amoxicillin and that inhibits a wide range of β-lactamases.
Although Augmentin’s approved indications were initially somewhat restricted, they were soon broadened to include a wide range of infections in all age groups as the product’s safety and efficacy were confirmed in clinical practice. Indeed, the product was so successful that the combination was awarded its own British Approved Name (BAN), co-amoxiclav. There are now many generic versions available, although clavulanic acid has never been combined with other β-lactam antibiotics in a commercial product for human use (it is also used in veterinary medicine), except for a handful of cephalosporin/clavulanic acid combinations marketed exclusively on the Indian subcontinent.
While the introduction of penicillin and other antibiotics in the 1940s is generally regarded as marking the beginning of the antibiotic era, specific antimicrobial products first entered commerce much earlier in the 20th century. Hoechst (now part of Sanofi) launched Salvarsan (arsphenamine) for the treatment of syphilis in 1910, for example. From the early days of antimicrobial therapy, resistance was recognized as a problem, with sulfonamide resistance emerging in the 1930s, and resistance to penicillins was recorded relatively soon after that class of drug entered clinical practice. Interestingly, phylogenetic studies have shown that antibiotic-resistant genes have been present in bacteria since long before the antibiotic era – for many millions of years in some cases.
By the 1980s, bacterial resistance had emerged not only to the first generation of penicillins but also to semisynthetic penicillins and cephalosporins. More recently, resistance has been recorded to the newer carbapenems, which were developed specifically to be less susceptible to the development of resistance. When Augmentin was introduced it raised the hope that the spread of resistance could be overcome, but that unfortunately has not been the case. Resistance to antibiotics now poses a major global threat, and we are at risk of entering a “post-antibiotic era.” According to the World Health Organization in 2014:
- resistance to carbapenems, which had become the treatment of last resort for life-threatening infections caused by Klebsiella pneumoniae, had spread to all regions of the world;
- resistance to fluoroquinolones, widely used to treat urinary tract infections caused by Escherichia coli, was also widespread; and
- treatment failure with third-generation cephalosporins, the treatment of last resort for antibiotic-resistant Neisseria gonorrhoeae, had been confirmed in Europe, Australia, North America, Japan and South Africa.
Exhibit 1
Scale Of The Antibiotic Resistance Problem

Centers for Disease Control & Prevention
In general, the level of antibiotic-resistant infections in individual populations correlates with the level of antibiotic consumption (in other words, the more antibiotics are used, the more common is antibiotic resistance), which suggests they are not being used correctly. (See Exhibit 1.) One possible strategy for limiting the spread of antibiotic resistance could be the more appropriate use of antibiotics. In the UK, at least, we are starting to see public awareness of this issue being raised, albeit via a rather excruciating publicity campaign.
Other possible strategies to limit antibiotic resistance include the development of new antimicrobials directed at existing molecular targets, and the identification of new targets for antimicrobial agents. Clearly, industry needs to do more in this respect. In 2014, President Barack Obama tried to kick-start research into new antibiotics with an executive order that, among other things, led to the creation of the Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria (PACCARB). In September 2017, PACCARB voted to adopt a draft report containing recommendations for incentivizing the development of therapeutics, diagnostics and vaccines to combat antibiotic resistance. Hopefully, these recommendations will be accepted and will have the desired effect. Still, it is ironic that 35 years after the first antimicrobial product designed to address the resistance problem was launched, the problem is now more pressing than ever.
Success Against HIV/AIDS
It has not all been bad news in the anti-infective field, however. Another big story in the 1980s was the emergence of AIDS, and the industry’s response to it. Although the first cases of human infection with what became known as human immunodeficiency virus probably occurred in the 1920s, AIDS was only recognized as a distinct clinical entity in the early 1980s, when multiple cases of a previously unrecognized syndrome came to the attention of health officials. The syndrome was characterized by the presence of one or more otherwise rare opportunistic infections such as Pneumocystis carinii pneumonia, and seemed to be confined, initially at least, to four groups of individuals: heroin addicts, homosexuals, hemophiliacs and Haitians.
The unusual combination of features initially gave no clue to the origin of the syndrome, until it was realized that all those affected shared one thing in common – a severely compromised immune system, which gave rise to the name Acquired Immune Deficiency Syndrome (AIDS). In 1983, Luc Montagnier’s group in Paris announced that they had isolated the virus responsible for AIDS, which they named lymphadenopathy-associated virus (LAV), while shortly afterwards, Robert Gallo's group in the US reported that they had characterized the causative agent as human T-lymphotropic virus type 3 (HTLV-III). In 1984 it was agreed that LAV and HTLV-III were one and the same virus, which was later renamed human immunodeficiency virus (HIV).
This was the signal for both the diagnostics industry and the pharmaceutical industry to set to work to develop diagnostic tests and therapeutic agents for HIV infection. In 1985, the FDA licensed two ELISA tests, from Abbott Laboratories Inc.and Electro-Nucleonics (now Alfa Wassermann SPA), for screening blood donations for HTLV-III, while a similar test based on the LAV isolate was developed by Genetic Systems. In 1987, the first diagnostic test became available when the FDA approved the use of Western blot testing for detecting HIV antibodies in the blood of suspected AIDS patients, and the same year it also approved the use of the reverse transcriptase inhibitor, zidovudine (azidothymine, AZT), for the treatment of HIV infection. The product, developed by GlaxoWellcome (now GSK) and marketed as Retrovir, was the first to be approved specifically for the treatment of HIV infection, less than four years after the virus was first identified, an outstanding achievement on the part not only of the researchers who developed it but also the regulators who approved it in record time.
Since then, of course, techniques for diagnosing HIV infection have improved considerably, and the number of antiretroviral drugs available has also mushroomed to include not only reverse transcriptase inhibitors but also protease inhibitors, integrase inhibitors, fusion inhibitors and chemokine receptor antagonists. Research continues: only recently, it was reported that Opdivo (nivolumab), a human monoclonal antibody developed by Ono Pharmaceutical Co. Ltd.and Medarex Inc.(part of Bristol-Myers Squibb Co.) as an anticancer, may be able to deplete dormant virus reservoirs in the tissues of patients with HIV, which up till now has been an obstacle to eliminating the virus from the body.
Just to underline the fact that the virus is not yet completely vanquished, some 36.7 million people worldwide are currently living with HIV/AIDS, and last year 1 million people died from AIDS-related illnesses, according to the Joint United Nations Programme on HIV and AIDS (UNAIDS). Nevertheless, the rapidity of the original response to the AIDS crisis demonstrates how effectively the industry can respond in an emergency.
Exhibit 2
HIV/AIDS In Numbers
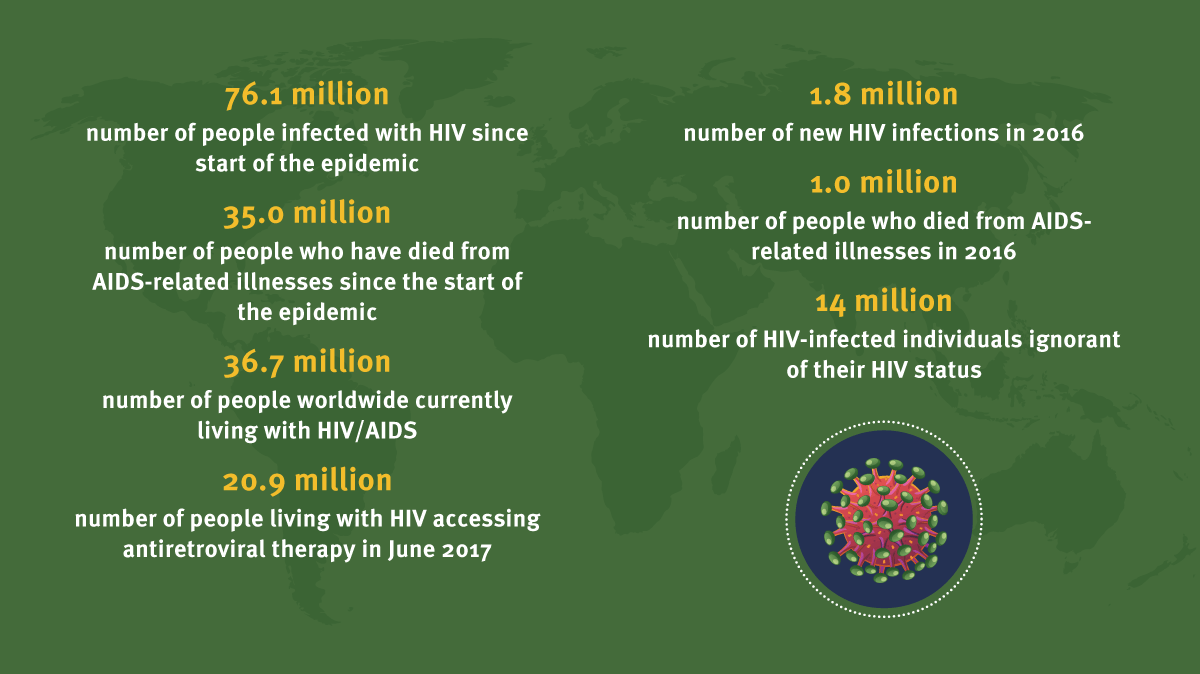
UNAIDS
The past 35 years have also seen products successfully introduced for the treatment or prevention of other viral diseases, including hepatitis C (which accounts for most cases of what was then known as non-A, non-B viral hepatitis) and human papillomavirus (HPV, thought to be responsible for most cases of cervical cancer). In the case of hepatitis C, the emergence of safe and effective treatments has brought a new problem, for payers at least: their cost. A 12-week course of Gilead Sciences Inc.'s Harvoni (ledipasvir + sofosbuvir), for example, can cost $94,500. Responses to these high prices have included use of an indication-specific budget, sliding price-volume agreements with manufacturers, an expenditure cap in combination with a creative taxation scheme and market access restrictions including strict patient-prioritization criteria. Although such measures have tended to restrict the availability of hepatitis C treatments in major markets, the product sector has shown meteoric growth since its inception in 2011. At the same time, however, the threat posed by other virulent viruses, such as Ebola or Marburg virus, remains.
Metabolic Disease Treatments
Turning aside from infectious diseases, major challenges remain in other areas. Diabetes is still a significant problem, especially in view of the prevalence of obesity in many populations. The 1980s saw a substantial amount of research into new treatments for diabetes, although these efforts were not to bear fruit until 1990, when Bayer AGlaunched the α-glucosidase inhibitor, Glucobay (acarbose), the first new primary therapy for diabetes since the introduction of the sulfonylureas and biguanides in the 1950s. Inhibition of α-glucosidase reduces the rate at which complex carbohydrates are digested, thus lowering blood sugar levels.
The emphasis changed in the 1990s, when new insulin analogs began to appear on the market. The first of these was Humalog (insulin lispro), Eli Lilly & Co.’s fast-acting human insulin analog that was designed more closely to mimic the body's insulin output in response to eating. Manufactured via recombinant DNA technology, Humalog made its debut in 1996. It was followed in 1999 by NovoLog (insulin aspart), a rapid-acting insulin analog developed by Novo Nordisk ASfor the treatment of types 1 and 2 diabetes. Then in 2000, Aventis (now Sanofi) launched Lantus (insulin glargine), a long-acting human insulin analog manufactured using recombinant DNA techniques in bacteria via the proinsulin route.
In the intervening years one interesting innovation in this area has been inhaled insulin, which was initially promoted as a more patient-friendly alternative to insulin injections. The first such product was Exubera, developed by Inhale Therapeutic Systems and launched commercially by Pfizer Inc. in 2006. But the market did not appear to be ready for such an innovation, and poor sales led to its being withdrawn in 2008. Inhale changed its name to Nektar Therapeuticsand turned its attention to other applications for its polymer conjugate technology platform. More recently, in 2015, MannKind Corp. launched Afrezza, a dry powder pulmonary formulation of synthetic human insulin for the treatment of type 1 and type 2 diabetes. That said, Lantus and Humalog continue to dominate the insulin market, although their position over the next few years could be threatened by the introduction of biosimilar versions.
According to Treatment: Diabetes Type 1, an April 2017 Datamonitor Healthcare report, there is currently a significant unmet need for non-insulin therapies in type 1 diabetes. Alongside insulin-based therapy, drugs with other mechanisms of action could be exploited to improve glycemic control in these patients, as well as reduce insulin dosing and associated side-effects. The existence of this unmet need is demonstrated by the fact that type 2 diabetes therapies are already used off-label in type 1 diabetes patients.
Progress Toward An Artificial Pancreas
Of course, injecting insulin is inconvenient and can only ever approximate normal physiological conditions. A long-term goal since even before the 1980s has been to develop an artificial pancreas that can respond to minute-by-minute variations in blood glucose and deliver the appropriate amount of insulin with minimal intervention by the patient. Such a device would greatly simplify glycemic control and, by leveling out peaks and troughs in blood glucose, would minimize the risk of diabetic complications.
Although a truly autonomous implantable artificial pancreas has yet to be produced, both insulin pump and glucose sensor technology have steadily improved over the years. Medtronic PLC’s MiniMed 670G system, for example, was approved by the FDA in September 2016 and is the world’s first hybrid closed-loop system that constantly self-adjusts to automatically keep the patient’s sugar levels in the correct range. It features SmartGuard technology, described by Medtronic as “one step closer to The Artificial Pancreas,” which provides advanced protection from hypoglycemic episodes. Nevertheless, for many type 1 diabetics, insulin injections look likely to be around for some time yet.
Few Novel Drugs For Obesity
There remains no known cure for either type 1 or type 2 diabetes. The latter is associated with obesity, whose prevalence is steadily increasing in developed countries and for which there also seems to be no cure on the horizon. The WHO calculates that worldwide obesity has nearly tripled since 1975 and that, in 2016, more than 1.9 billion adults were overweight, of whom more than 650 million were obese.
There are currently nearly 40 compounds in clinical trials for the treatment of obesity, according to Informa Pharma Intelligence's Pharmaprojects. Receptors for glucagon and glucagon-like peptide-1 (GLP-1) seem to be popular targets for potential anti-obesity products. However, new product introductions over the past few years have been relatively few, and several of those have been new combinations or formulations of older products.
Just three anti-obesity products launched in the past 30 years stand out for having a novel mode of action, but none of them is ideal. The first, launched in 1998, was Roche’s Xenical (orlistat), which acts by inhibiting pancreatic lipase, thus inhibiting fat absorption. The fact that Xenical does not act via an effect on the central nervous system is a potential advantage in both therapeutic and marketing terms, but it can have unpleasant gastro-intestinal side-effects which can lead to poor patient compliance. Orlistat is now available over-the-counter as Alli.
Nearly 15 years after the introduction of Xenical, Novo Nordisk launched the GLP-1 agonist Saxenda (liraglutide): it acts on the brain to simulate the effect of endogenous GLP-1 to depress appetite. Its major drawback is that it must be administered subcutaneously, which limits its usefulness (it is also relatively expensive). More recently, Arena Pharmaceuticals Inc.introduced Belviq (lorcaserin hydrochloride), a first-in-class 5-HT2C agonist as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with at least one weight-related comorbid condition. However, there have been reports that the effectiveness of Belviq is lower than that of some other treatments.
While an effective pharmacological treatment for obesity remains elusive, there has recently been significant progress in the medtech area, particularly in the area of minimally invasive bariatric devices, a market that is growing by more than 15% a year.
One of the earliest drugs used to produce weight loss was amphetamine, which has anorectic properties, and older anti-obesity products tended to act via a similar central stimulant effect. Such products had obvious abuse potential but, despite the introduction of some innovative new anti-obesity products over the past couple of decades, the ideal therapeutic agent has yet to be found. A handful of potential anti-obesity agents are currently in advanced clinical testing, including: an orally active dopamine, noradrenaline and 5-HT re-uptake inhibitor, under development by Saniona AB; an oral angiogenesis and matrix metalloproteinase inhibitor targeting adipose tissue, under development by AngioLab; and a chewable plant-derived, carbohydrate hydrolyzing inhibitor, under development by Boston Therapeutics Inc.
While an effective pharmacological treatment for obesity remains elusive, there has recently been significant progress in the medtech area, particularly in the area of minimally invasive bariatric devices, a market that is growing by more than 15% a year, according to Minimally Invasive Weight Loss Devices Market, a July 2017 report from Informa's Meddevicetracker. For many years, this sector was dominated by laparoscopic adjustable gastric banding devices, but these are now being overtaken by intragastric balloons, sales of which are expected to grow by over 30% a year between now and 2021. Intragastric balloons, which were first approved in the EU in 1997 and by the US FDA as recently as 2015, are particularly suited for patients with moderately increased BMI (basal metabolic index, a measure of overweight) for whom more invasive procedures such as sleeve gastrectomy are not appropriate. (Also see "Bariatric Devices: Intragastric Balloons To Eclipse Gastric Banding" - In Vivo, 18 Oct, 2017.)
Market leader in this sector is Apollo Endosurgery Inc.’s Orbera system: other players include ReShape Medical Inc.(formerly known as EnteroMedics) and Obalon Therapeutics Inc. Intragastric balloons are only a short-term solution to appetite control (they are removed after six to 12 months) but are generally well tolerated, although there have been some concerns about the risk of over-inflation.
Lowering Lipids
To return to the subject of lipid metabolism for a moment, one class of drug whose use is widespread now but that was virtually unknown 30 years ago is the statins. I even take one myself, as does President Trump, apparently, so I am in good company. Statins lower LDL cholesterol levels significantly and cause a moderate reduction in HDL-cholesterol as well as having other beneficial effects in dyslipidemias such as reducing inflammation, C-reactive protein levels, plaque size and clot formation. They have repeatedly been shown to lower the occurrence of atherosclerotic cardiovascular disease events (they may also be able to reduce mortality from certain types of cancer), and thus have become, and seem likely to remain, the standard first-line treatment for hypercholesterolemia.
The first statin to be marketed was Merck & Co. Inc.’s Mevacor (lovastatin) in 1987, but it was Lipitor (atorvastatin), launched by Parke-Davis (then part of Warner Lambert but now a part of Pfizer) exactly 20 years ago, in 1997, that really set this market alight. Thanks to clever marketing, Lipitor rapidly outsold every other pharmaceutical product on the market, and even now is still the world’s biggest selling prescription drug of all time, when lifetime sales of $148,744 million are considered.
The statin class is now widely genericized, which has depressed prices: even so, the global market for these products is currently worth somewhere in the region of $12 billion annually – not bad for a market that didn’t exist 30 years ago.
One of the pioneers of magnetic resonance imaging, Peter Mansfield, PhD, who died in 2017, once commented that when the first human NMRI experiments were carried out, he was concerned that the magnetic field would completely erase the subject’s memory, rather like a tape recording can be erased with a magnet. Fortunately, that did not happen.
Advances In Oncology
No review of this nature would be complete without a mention of the tremendous progress there has been in the treatment of cancer. Many types of cancer are now considered, like HIV infection, to be chronic but manageable conditions rather than incurable and often fatal. At the beginning of the 1980s, cancer therapy depended largely on surgery and the use of cytotoxic agents – antimetabolites, alkylating agents, plant-derived agents such as the vinca alkaloids, antitumor antibiotics and so on. While these types of drug still have a role to play, cancer treatment is now much more focused on the cytogenetic mechanisms underlying the cellular changes in the disease.
Between 1980 and 1990, only a handful of new anticancer agents reached the market, although the trend toward more specific types of therapy was already apparent, with drugs targeting interferon or interleukin receptors being introduced, for example. Contrast that with the period 2007–17, when over 150 new anticancer products appeared on the market, many with new immunological targets. (See Exhibit 3.)
Exhibit 3
Biological Targets Of Selected New Anticancer drugs, 2007–17
|
Target |
Number of Products |
|
erb-b2 receptor tyrosine kinase 2 |
11 |
|
Pinase insert domain receptor |
8 |
|
Membrane-spanning 4-domains, subfamily A, member 1 |
8 |
|
Epidermal growth factor receptor |
7 |
|
v-kit Hardy Zuckerman 4 feline sarcoma viral oncogene homolog |
6 |
|
fms-related tyrosine kinase 1 |
5 |
|
Platelet-derived growth factor receptor, alpha polypeptide |
5 |
|
ret-proto-oncogene |
5 |
Pharmaprojects | Pharma Intelligence, 2018
At the same time, the diagnosis of cancer has progressed considerably. In the early 1980s, a new imaging technique called nuclear magnetic resonance imaging (NMRI) was introduced that revolutionized the diagnosis of cancer and many other conditions. Although the technique was based on the principle of nuclear magnetic resonance, the word “nuclear” was later dropped for fear it would frighten patients, leaving just the “MRI” acronym we know today. One of the pioneers of magnetic resonance imaging, Peter Mansfield, PhD, who died in 2017, once commented that when the first human NMRI experiments were carried out, he was concerned that the magnetic field would completely erase the subject’s memory, rather like a tape recording can be erased with a magnet. Fortunately, that did not happen.
Science Still Matters
Over the years, growth in some product sectors has followed a ballistic trajectory, only to decline as medical practice evolves. In 1976, Smith Kline & French in the UK launched a new treatment for peptic ulcer, the H2 antagonist Tagamet (cimetidine). At that time, a peptic ulcer was a painful, debilitating, even life-threatening condition for which the only drug treatment, antacids, provided at best limited relief. Other treatments used ranged from adoption of a bland diet to truncal vagotomy to restrict acid secretion in the stomach.
That all changed with the introduction of Tagamet, which proved to be a much more effective way of inhibiting gastric acid secretion and which by the 1980s had become the world’s first “blockbuster” drug with annual sales of more than $1 billion. Tagamet blocks acid secretion in the stomach by antagonizing H2 receptors, and was developed by a team led by Nobel Prize winner James Black, later Sir James Black, who used rational drug design to create a molecule that specifically blocked these receptors, an approach he had previously used successfully in the development of the first β-blocker, propranolol (ICI’s Inderal).
Within a few years, Tagamet sales were eclipsed by those of Glaxo’s rival product, Zantac (ranitidine). Other H2 antagonists from other manufacturers followed, but their success was cut short by the arrival of a new class of acid-inhibiting compounds, the proton pump inhibitors (PPIs), of which the first was AstraZeneca PLC’s Losec (omeprazole).
All the while these new treatments for peptic ulcer were being introduced, evidence was mounting that many cases of peptic ulcer were in fact caused by Helicobacter pylori, a Gram-negative bacterium that is typically found in the upper gastrointestinal tract (some cases, though, do have other causes such as frequent use of NSAIDs, alcohol consumption, smoking, etc). Treatment today usually consists of a PPI to lower acid secretion together with an appropriate antibiotic to target the H. pylori infection.
The move away from H2 antagonists for treating peptic ulcer was not the end of the story for that class of medicine. In the mid-1990s, Tagamet was one of several products that were part of a new wave of Rx-to-OTC switching, driven by the need to extend the product’s life cycle in the face of changing prescribing habits and by the push by health systems to shift the cost of treating minor ailments onto the patient (OTC cimetidine is used to prevent and treat the symptoms of heartburn associated with acid indigestion). Patent protection of cimetidine has since expired, and GSK has disposed of its Tagamet (and Zantac) assets.
Pharma's Reputation
One theme that has recurred over the past 35 years (and probably longer) is that of the public perception of the industry. Many pharmaceutical companies were founded with altruistic aims and a commitment always to act in the interest of patients. However, as many of these companies have grown to become major multinational corporations, they have become increasingly susceptible to the expectations and demands of the financial markets and are now often perceived to act in the interests of shareholders in preference to those of patients.
This problem was epitomized by the outcry that arose when the first antiretroviral drugs were marketed. Manufacturers kept prices high, even in developing countries where ironically most HIV infections occurred, and resisted attempts to permit the use of generic versions from low-cost countries such as India. Such behavior attracted much criticism, and the companies eventually capitulated and lowered their prices, while President Bill Clinton issued an executive order to prevent the Office of the US Trade Representative from seeking trade sanctions against poor countries that tried to gain access to generic versions of anti-HIV drugs (as they were permitted to do under World Trade Organization rules).
Some of the challenges currently facing the health care industry are the same ones that were faced decades ago – only the means used to tackle them has changed.
The industry eventually recovered from the damage done to its reputation by the debacle, but since then it seems, whether through arrogance or naivety, to have blundered into several other crises in public confidence. Among the practices that have attracted public condemnation of the industry are making its products unaffordable to many patients, even in wealthy countries like the US; indulging in mega-mergers that, while they may lead to a lower tax burden, an improved bottom line and amplified executive pay, do little for patients or indeed their own workforce; and indulging in behavior that has attracted financial sanctions, such as inappropriate marketing, promotion of off-label indications and misleading direct-to-consumer advertising. The failure to tackle the problem of antibiotic resistance is another oft heard criticism. The actions of companies like Turing Pharmaceuticals AGand its then CEO Martin Shkreli, which acquired the toxoplasmosis drug Daraprim (pyrimethamine) and then hiked the price by over 5,000%, did not help.
Lessons Learned
There are a couple of important lessons to be learned from all of this. First, although much changes with the passage of time, much also remains the same. Some of the challenges currently facing the health care industry are the same ones that were faced decades ago – only the means used to tackle them has changed. Second, the future is exceedingly difficult to predict with any accuracy, and even the past is no guide to the future. Markets can appear from nowhere almost overnight: the statins have already been mentioned – phosphodiesterase type 5 inhibitors, used in the management of erectile dysfunction and shortly to become available OTC we are told, is another.
Also apparent is the growing convergence between the pharma and medtech sectors. The story of the discovery of HIV is a good example: once the virus had been identified the way was open to develop antiviral agents with which to treat infected individuals, but these would have had limited use without the availability of diagnostic tests to identify those patients. More recently, companion diagnostics have emerged as a vital component of many new, high-tech pharmaceutical product offerings. Perhaps such convergence will turn out to be the catalyst for successfully addressing some of the outstanding challenges facing the health care sector.
It is sometimes said that the pharmaceutical industry has taken all the low-hanging fruit in terms of treating disease, and that it will be increasingly difficult to address those diseases that remain to be conquered. It therefore seems likely that there will be greater emphasis on the prevention of disease, rather than its treatment. Indeed, this trend is already apparent in the development of HPV vaccines to prevent cervical cancer, and greater emphasis on prevention may well turn out to be a partial answer, at least, to the management of infectious diseases caused by antibiotic-resistant bacteria. Alzheimer’s disease, which we have not mentioned previously but for which there are no quick fixes, may also ultimately be beaten by the adoption of appropriate preventive measures (which of course also remain to be discovered).
New and emerging technologies like artificial intelligence, machine learning and data mining will also have an important role to play in the continuing fight against human disease. The next 35 years should be even more exciting than the last.
