Rising From The Ashes Of 2020: ‘We Must Not Waste The Learning,’ Says Dame Sally
Executive Summary
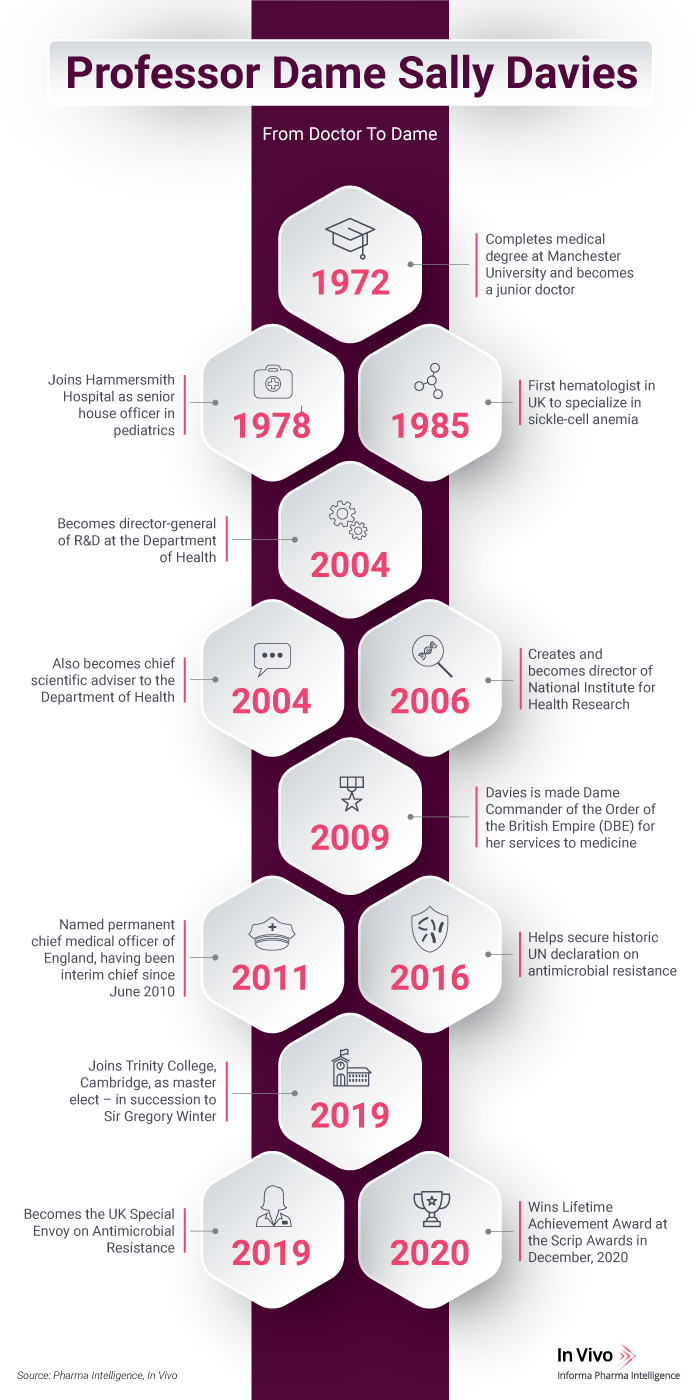
UK special envoy on antimicrobial resistance Professor Dame Sally Davies, who is also the former chief medical officer for England, talks to In Vivo about the challenges of 2020, the importance of a strong working relationship between the public and private sectors, and reaching a turning point on AMR.
Professor Dame Sally Davies, the most recent winner of Scrip’s Lifetime Achievement Award, is the UK’s special envoy on antimicrobial resistance. In an exclusive interview with In Vivo she discussed the incredible events of 2020 and what lies ahead, the public’s evolving relationship with health care and the next steps against antimicrobial resistance (AMR).
In her role as special envoy, Davies works with governments around the world on AMR – “It’s a slow pandemic that's growing.” She is also the master of Trinity College in Cambridge – the first woman to hold this position. Previously, Davies was chief medical officer for England and chief medical adviser to the UK government from March 2011 to September 2019, having held the post on an interim basis since June 2010. Other highpoints in a long career have included being accorded a seat on the United Nations interagency coordination group for AMR, where she was one of the co-conveners. And recently she was appointed to the UN Global Leaders Group for AMR. “I’ve been at this for years,” she said light-heartedly.
“I've been working with industry ever since I moved into running research and development for the NHS in 2004,” Davies noted. Back in 2000, she recalled, she spent time on secondment to UK big pharma GlaxoSmithKline plc, in order to better understand clinical research and development in the life sciences sector. Getting the inside view of drug R&D rather than the public sector and NHS perspective.
Earlier in her career, Davies was a clinical researcher in Brent, North West London, looking after patients with sickle cell disease. She then shifted gears to focus on R&D within the NHS and “persuaded ministers to allow me to set up the National Institute for Health Research with my team and many wonderful people.” The NIHR launched in 2006. “We put in place the infrastructure for experimental medicine, the biomedical research centers and the managed clinical research network of the NIHR.” Davies added that what the center had achieved so far was “very impressive”.
In 2011, Davies was responsible for linking NIHR funding to the Athena SWAN award, a national charter mark that recognizes the advancement of gender equality. “In other words, I said you have to support women in science … or we won't be funding you.”
Importance Of Collaboration
In recalling some of her many career highlights, Davies said she was pleased with her achievements so far, but impatient for more. “We've made good progress. I'm happy that I've helped put things on the map … and we've made a difference. It's just that I wanted everything to happen yesterday, and it's not there yet.”
Whether it is the global challenge of antibiotic resistant diseases or gender equality in the business world, Davies said progress was being made all around in the health care sector. “I'm a glass-half-full person, we will get there. What you'll see is a silver thread through all my work where I am working in collaboration with whomever I need to work with. So, I work with governments, I work with the NHS, but I also work with the life sciences sector. We all need the same answers for the same systems to work. It's not very difficult, where our agendas overlap, to respect each other and work together.”
Davies noted some of the unexpected collaborative efforts in 2020 that came about during the COVID-19 pandemic, such as the Access to COVID-19 Tools (ACT) accelerator. ACT-Accelerator is a ground-breaking global collaboration to accelerate development, production and equitable access to COVID-19 tests, treatments and vaccines. Launched in April last year, the ACT-Accelerator has supported coordinated global efforts to develop tools to fight COVID-19. It brings together governments, scientists, businesses, civil society, and philanthropists and global health organizations (such as the Bill & Melinda Gates Foundation, CEPI, FIND, Gavi, The Global Fund, Unitaid, Wellcome, WHO and the World Bank). “With significant advances in research and development by academia, private sector and government initiatives, the ACT-Accelerator is on the cusp of securing a way to end the acute phase of the pandemic by deploying the tests, treatments and vaccines the world needs,” the group said in 2020. A total of $5.8bn in funding had been contributed to the ACT-Accelerator as of January 2021.
Davies said experience from COVID-19 had re-established the importance of the private sector in health care. “The private sector can do some things that the public sector cannot. We couldn't produce vaccines, but we can mobilize to get them to people. We, in the public sector, don't produce diagnostics, nor do we do manufacturing. So, we've seen the value of the private sector.”
One lesson Davies highlighted as something she had learned throughout her career, was the “importance of not going too fast, but getting the right speed.” And not leaving people behind in the process. At Trinity College, Davies has launched The Trinity Challenge. It is a “unique coming together of private sector and academia to make sure we're better prepared for future pandemics,” she noted. Through this activity, Davies said she had “learned a lot more about the value of data and technology and how you can put them together.” Some of the companies involved include GSK, Facebook, Google, and Microsoft.
The Trinity Challenge
The Trinity Challenge coalition is focused on three core activities to improve the use of data and analytics in response to health emergencies:
-
Setting new global challenges to the world;
-
Facilitating unique collaboration amongst the collation to improve outcomes;
-
Strengthening the data, analytics and learning ecosystem for global public health.
It is a call to action for the world’s best and brightest minds to contribute ideas and innovations, with £10m ($13.6m) in awards for the best Challenge Teams.
Ideas and concepts submitted to the challenge must address at least one of three areas in which to improve the world’s approach to pandemic prevention and control through data and analytics: identification, response and recovery.
Despite the western world being “slow to react” to the threat of COVID-19, Davies praised the speed of vaccine development. “I never dared dream that we'd be putting vaccines into people at this stage,” she said. This success, with the first COVID-19 vaccine getting approval just a few months after the disease was declared a pandemic by the World Health Organisation, is because of strong investment in science. “It’s all about having invested in research in the past so that you can pivot it,” Davies said. “Whether it's the Oxford/AstraZeneca, where Oxford had been funded by the UK vaccines network, among others, to work on a MERS vaccine, or whether it's
Moderna, Inc. or who could pivot because they were working on mRNA viruses for cancer. It shows the value of long-term investment in science, at the very basic level, and in the infrastructure for it.”
Raising The Bar
Still, this incredible success by biopharma companies and academic partners to get vaccines to market so quickly has created high expectations for the future of drug development. Manufacturers have “set themselves standards that they're going to find hard to keep up with, but they've got to,” Davies said. “We must, all of us, go on investing in the science and the infrastructure, so that when we get a threat we're well prepared,” she said. “And we need to think about those wider preparedness benefits against emerging threats.”
Davies added that there was still a need to be investing in new technologies and different methods for producing vaccines. She also suggested the biopharma industry should look again at its efforts against AMR. “If you can work this fast and effectively in collaboration, we need a sustainable pipeline of antibiotics to come together.” She added that the AMR Action Fund was a great move, “but we need the big companies to go back into it.”
The concept of the AMR Action Fund was developed in collaboration with WHO, the European Investment Bank and the Wellcome Trust. Launched in July 2020, the initiative includes more than 20 biopharmaceutical companies that have pledged to invest nearly $1bn, with the aim of bringing 2-4 new antibiotics to market by 2030. The AMR Action Fund will invest in small companies developing innovative antibacterial treatments. It will forge partnerships with institutions and philanthropic organizations, development banks, and multilateral organizations to strengthen and accelerate antibiotic development. It will also work with governments to ensure there is a healthy flow of new antibiotics to fight superbugs.
Public Engagement In Health Care
“We have a responsibility to learn from this pandemic. To be better prepared, with agile manufacturing and picking back up the priorities that were dropped [in infectious disease research],” Davies said. With the announcement of a new UN Global Leaders Group on AMR, she expects to see more action in the coming months and years. “I'm determined that we move from talk to some action. And we'll do it in collaboration with the private sector, which will be play major part. We've got to show that we can learn from COVID and rise up from the ashes and not waste the learning.”
Davies noted that with AMR, it was difficult to find the patient face, and this had hindered messaging on the issue. In comparison, “Cancer charities around the world are beginning to recognize that this matters to them to provide a face. But doctors and nurses don't say to patients, ‘This is an antibiotic-resistant bacterium, so we're changing treatments,’ nor do they say to relatives when a person dies of sepsis that it was untreatable, a resistant infection.”
To progress in the fight against AMR, “we need to move to much more openness about when it's happening, because it is there, in all our health systems, we need … that patient face,” she said. “And we also must be much more conversational about it, without too much scientific mumbo jumbo.”
Positive impacts from the ongoing pandemic include a better general understanding by the public, especially children, of infection prevention and control, hand washing, and hygiene. “All of these things help to mitigate other infections, whether it's flu or bacterial infection,” Davies noted. She also highlighted the public’s openness to vaccines. “There seems to be little disagreement that the vaccines are important in our response to COVID.” This could have a broader impact. “Clearly, if you vaccinate people against flu, not only do fewer get flu cases, there is also less transmission. With fewer getting flu, you'll have less bacterial infection and less AMR. These all are interlinked.” While there will still be some vaccine hesitancy, Davies hopes that overall people will see the long-term benefits about prevention, preparedness and vaccination.
Market Failure For Antibiotics
The pharmaceutical industry stepped away from novel antibiotic development in recent years, in favor of more lucrative and specialized therapeutic areas. One of the major hindrances to antibiotic development is the lack of economic models to suit the development of brand-new drugs that need to be rationed in supply, under antibiotic stewardship.
“I'm very sympathetic,” said Davies. “I do understand that we have a market failure. But the world has moved on and we still have a problem pulling new antibiotics through the pipeline. That's why I'm thrilled about the AMR Action Fund.” She added that the fund represents the life sciences sector coming together to do something about AMR.
Davies is also happy to see governments taking action on this problem. She cited the UK, where the government is trialling a ‘Netflix model’ for novel antibiotics. The subscription-style approach will offer participating drug makers an upfront payment in exchange for access to their antibiotics. This differs from the current system, which bases payments on the volume of antibiotics sold to the NHS. Initially, the health service is working with two antibiotics developers. (Also see "‘World’s First’ Antibiotic Incentive Scheme Kicks Off In UK" - Pink Sheet, 18 Jun, 2020.)
“We're getting more good news stories about work against AMR from around the world,” Davies added. For example:
-
The Indian government in early 2020 consulted on draft legislation to place limits on antibiotic residues from pharmaceutical manufacturing;
-
Chile has focused on reducing its antibiotic usage in fish farming, as has Norway;
-
While total antibiotic consumption in England fell by 6% between 2014 and 2017.
Davies also cited the EU's new pharmaceutical strategy, which is committed to introducing novel pull incentives for antibiotic development.
She said industry had a right to question the issues around economic models for new antibiotics: “There's a market failure, but I think there's quite a lot of discussion and action as well.” She said pharma should not give up working with the public sector to try and find the right solutions. “We're not there yet. But I hope we are at the beginning of the end of this conversation – or the end of the beginning.”
Rebuilding In 2021
The question of looking ahead into the coming year leads to another: “Can we rebuild better after COVID-19?” The pandemic has “exposed and exacerbated inequalities and vulnerabilities in the UK and across the world,” Davies said.
She said the focus when rebuilding in 2021 should be on equal and inclusive societies. Davies recently published a book, with Jonathan Pearson-Stuttard, titled Whose Health Is It, Anyway?, which focuses on how health care services import illness rather than exporting health. It outlines why health is the most untapped opportunity for prosperity and happiness in the 21st century, individually and jointly as whole nations.
“As we go forward, not only must we work on vaccine hesitancy, and make sure the vaccines get to people, but they've got to get everyone. They've got to get to low-income countries equally as to the rich countries. And we've got to make sure that we sort out AMR as we go forward. We've got to make sure that our supplies of antibiotics and anti-infectives are sustainable, and that there's good access, because not all drugs are licensed and available in all countries – and not all can be afforded.”