Revealed: In Vivo's 2024 Rising Leaders
30 people to watch from across the life sciences sector
VIEW NOWIn Vivo is part of Pharma Intelligence UK Limited
This site is operated by Pharma Intelligence UK Limited, a company registered in England and Wales with company number 13787459 whose registered office is 5 Howick Place, London SW1P 1WG. The Pharma Intelligence group is owned by Caerus Topco S.à r.l. and all copyright resides with the group.
This copy is for your personal, non-commercial use. For high-quality copies or electronic reprints for distribution to colleagues or customers, please call +44 (0) 20 3377 3183
Printed By
The FDA and EMA have shared perspectives on the parallel scientific advice pilot for complex generics. Now industry stakeholders discuss whether transatlantic convergence is possible or merely a good idea on paper.
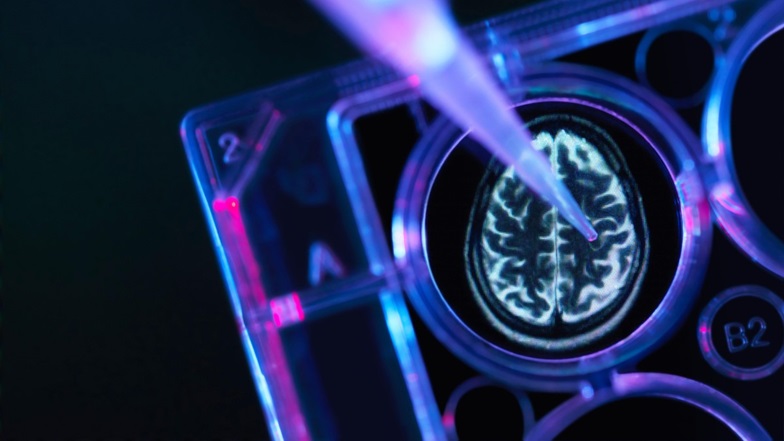
Can neuroscience be precise? Peter Fang, worldwide VP of neuroscience, explains J&J's approach to precision medicine in Alzheimer’s disease, depression and other neurological conditions.

The journey to Net Zero, described as ‘the defining issue of our time,’ will get harder in the coming decade. Failure to keep up the pressure will result in more long-term health conditions, increasing deaths and higher costs. The UK NHS is a Net Zero exemplar globally, but without a systemic approach, its compliance efforts could stutter.

Recent US government initiatives and growing investor interest are important for developing female-specific therapeutics, but funding is not yet where it should be.
With a reliable source of revenue in Arikayce and two key pulmonary late-stage trials reading out, Insmed aims to keep its commercial momentum going.
Arun Krishna reveals portfolio plans for best-selling therapy Tagrisso, and its other activities to prevent, screen and diagnose lung cancer faster.

Last year saw a drop in series A rounds. To increase the chances of fundraising success, new companies should engage earlier and align with potential financial backers.
CEO Hanadie Yousef leads Juvena Therapeutics in utilizing an AI-based platform that analyzes cell-secreted proteins to identify potential therapeutics. One of their first near-clinical assets could complement GLP-1s.
Eledon Pharmaceuticals’ CD40 Ligand blocker has yielded impressive results for post-transplant immunosuppression and cutting-edge transplant doctors have also used it for pig-to-human kidney and heart transplants, opening up the field of xenotransplantation.

In this episode of the In Vivo podcast, Eric Finzi, CEO of Healis Therapeutics, discusses how his company is targeting major depression, post-traumatic stress disorder and other mental illnesses using facial botox injections.
Can neuroscience be precise? Peter Fang, worldwide VP of neuroscience, explains J&J's approach to precision medicine in Alzheimer’s disease, depression and other neurological conditions.

ImproveWell has won awards for its software technology that allows health care staff at the sharp end of patient care to report on problems as they happen. The outcome is a real-time learning process that contributes to improved operational efficiencies for health systems, says In Vivo Rising Leader Lara Mott.

Leadership succession is proceeding within South Korean pharmaceutical corporations such as Daiichi Sankyo Korea, Astellas Pharma Korea, Kyowa Kirin Korea as well as MSD Korea.
With an engineer’s mind, Jason Park, CEO of Empress Therapeutics, has a unique take on problem solving for fundraising and the value of being a platform company focused on small molecules.

Addie MacGregor, at the Association of British HealthTech Industries, guides and advises an industry eager to comply but in search of direction, on matters of environmental sustainability for health care technology manufacturers.

ImproveWell has won awards for its software technology that allows health care staff at the sharp end of patient care to report on problems as they happen. The outcome is a real-time learning process that contributes to improved operational efficiencies for health systems, says In Vivo Rising Leader Lara Mott.

The CEOs of bluebird bio and CRISPR Therapeutics hope to inspire investor confidence that their gene-based therapies for sickle cell disease and beta thalassemia will be widely reimbursed in the US and EU.

The FDA and EMA have shared perspectives on the parallel scientific advice pilot for complex generics. Now industry stakeholders discuss whether transatlantic convergence is possible or merely a good idea on paper.

The journey to Net Zero, described as ‘the defining issue of our time,’ will get harder in the coming decade. Failure to keep up the pressure will result in more long-term health conditions, increasing deaths and higher costs. The UK NHS is a Net Zero exemplar globally, but without a systemic approach, its compliance efforts could stutter.
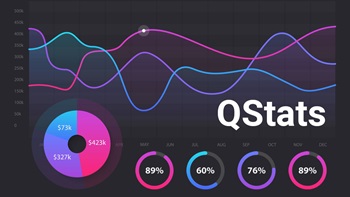
During Q1, biopharmas brought in an aggregate $30.1bn in financing and device company fundraising totaled $2.8bn; while in vitro diagnostic firms and research tools players raised $724m.

Could health sector players encounter issues similar to those facing Tesla in China, a country which virtually saved the electric vehicle maker but where it is now facing challenges? Are there any lessons to be learned from a success story under China's volume-based procurement scheme? A partner at EY looks at these and other issues.

Two $1bn+ alliances were penned in March. In the top alliance by deal value, Merus NV will use its Triclonics anitbody platform to discover new dual tumor-associated antigens targeting trispecific antibodies for Gilead Sciences. Merus will lead early-stage research activities for two programs, with an option to pursue a third. Gilead will have the right to license programs developed under the collaboration after the completion of select research activities. If Gilead exercises its option to license any such program from the collaboration, Gilead will be responsible for additional research, development and commercialization activities for such program. The deal could be worth up to $1.5bn for Merus.
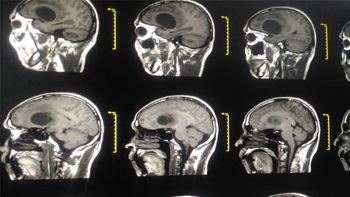
Glioblastoma carries a grim prognosis. The biopharma industry is trying to improve patient outcomes, with 120 drugs in the pipeline and two anticipated approvals this year.
CEO Hanadie Yousef leads Juvena Therapeutics in utilizing an AI-based platform that analyzes cell-secreted proteins to identify potential therapeutics. One of their first near-clinical assets could complement GLP-1s.
From X-ray film in 1936 to the world’s first digital X-ray system in 1983, Fujifilm has a long heritage in medical diagnostics.
During Q1, biopharmas brought in an aggregate $30.1bn in financing and device company fundraising totaled $2.8bn; while in vitro diagnostic firms and research tools players raised $724m.
ImproveWell has won awards for its software technology that allows health care staff at the sharp end of patient care to report on problems as they happen. The outcome is a real-time learning process that contributes to improved operational efficiencies for health systems, says In Vivo Rising Leader Lara Mott.
Eledon Pharmaceuticals’ CD40 Ligand blocker has yielded impressive results for post-transplant immunosuppression and cutting-edge transplant doctors have also used it for pig-to-human kidney and heart transplants, opening up the field of xenotransplantation.
In this episode of the In Vivo podcast, Eric Finzi, CEO of Healis Therapeutics, discusses how his company is targeting major depression, post-traumatic stress disorder and other mental illnesses using facial botox injections.
Could health sector players encounter issues similar to those facing Tesla in China, a country which virtually saved the electric vehicle maker but where it is now facing challenges? Are there any lessons to be learned from a success story under China's volume-based procurement scheme? A partner at EY looks at these and other issues.
The CEOs of bluebird bio and CRISPR Therapeutics hope to inspire investor confidence that their gene-based therapies for sickle cell disease and beta thalassemia will be widely reimbursed in the US and EU.
Leadership succession is proceeding within South Korean pharmaceutical corporations such as Daiichi Sankyo Korea, Astellas Pharma Korea, Kyowa Kirin Korea as well as MSD Korea.
The FDA and EMA have shared perspectives on the parallel scientific advice pilot for complex generics. Now industry stakeholders discuss whether transatlantic convergence is possible or merely a good idea on paper.
Recent US government initiatives and growing investor interest are important for developing female-specific therapeutics, but funding is not yet where it should be.
Favorable UK biopharma investment trends have been reported by the BioIndustry Association, but can medtech and healthtech share in the positivity? A roundtable at the BioWales in London 2024 event framed the outlook for non-pharma players.
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.