Market Access
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Helping Organizations Deliver On KPIs And Giving Staff A Voice
ImproveWell has won awards for its software technology that allows health care staff at the sharp end of patient care to report on problems as they happen. The outcome is a real-time learning process that contributes to improved operational efficiencies for health systems, says In Vivo Rising Leader Lara Mott.

As Casgevy, Lyfgenia Launches Proceed, CRISPR And bluebird bio CEOs Reassure
The CEOs of bluebird bio and CRISPR Therapeutics hope to inspire investor confidence that their gene-based therapies for sickle cell disease and beta thalassemia will be widely reimbursed in the US and EU.

FDA-EMA Advice Pilot For Complex Generics: What Does Industry Have To Say?
The FDA and EMA have shared perspectives on the parallel scientific advice pilot for complex generics. Now industry stakeholders discuss whether transatlantic convergence is possible or merely a good idea on paper.

The Sustainability Stakes Are Rising: ‘We Cannot Recycle Our Way Out Of This’
The journey to Net Zero, described as ‘the defining issue of our time,’ will get harder in the coming decade. Failure to keep up the pressure will result in more long-term health conditions, increasing deaths and higher costs. The UK NHS is a Net Zero exemplar globally, but without a systemic approach, its compliance efforts could stutter.

Coming Of Age: Pharma’s Influencer Marketing Matures
Influencer marketing has emerged as a potent tool for many industries and is a sector set to be worth $22bn in its own right by 2025.

An Ecosystem For Medtech Funding And Innovation Support – BioWales 2024
Creo Medical and Clinithink are among the healthtech innovators who have benefited from funding and advisory support offered by the Development Bank of Wales. All three gave a take on the current funding environment at BioWales in London 2024.

Moving Pharma Execs Out Of The Shadows Is Key To Building Public Trust
2024 Rising Leader, Jack O’Meara, says the industry must ‘promote personalities’ when building new biotech businesses to gain the trust of investors and the public.

Podcast: Identifying The 80% Of Undiagnosed And Misdiagnosed Patients
Vibhor Gupta, director and founder of Pangaea Data, talks to In Vivo about the value of identifying the 80% of patients with undiagnosed or misdiagnosed conditions, and how his company is addressing this need.
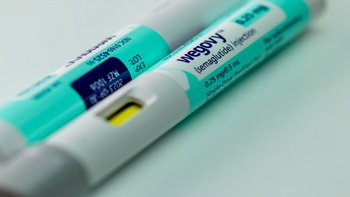
COVID To GLP-1: Catalent Plants Ride With Novo To Next Public Health Crisis
A complex transformation is underway as client proposes to acquire CDMO facilities that vaccinated the world against the COVID-19 pandemic for pivot to the obesity epidemic.

FTC’s Salvos Against Biopharma To Reverberate Through 2024
The Federal Trade Commission broke new ground last year in its opposition to M&A transactions and challenge of Orange Book patent listings. The biopharma community is waiting to see if deals will face similar hurdles in 2024 and whether there will be legal battles if manufacturers of drug-device combination products decline to delist their patents. Researchers advocate that the FTC extend its inquiry to device patents on GLP-1 receptor agonists, including Wegovy and Ozempic.

2024 Radar: UK Medtechs Up The Pace On Sustainability And Assess AI Potential
Three UK medtech companies’ views on how their businesses will be influenced by external factors in the coming year.

Times Of Unprecedented Change: German Medtech Readies For Challenging 2024
German medtech and health care are going through a period of unprecedented change and modernization, with hospital reform on the agenda and integration of digitized functionality happening across the sector. The EU Medical Device Regulation casts a long shadow over the medtech industry and will result in a shake out among companies and products, as In Vivo learnt at Medica 2023.

Why Did The European Commission Decide All Antimicrobials Should Be Rx-Only?
The European Commission responds to questions regarding its decision to include OTC antivirals and antifungals, including commonly used products like thrush and cold sore creams, in the expanded prescription-only requirements for antimicrobials, as part of proposed measures to combat antimicrobial resistance.

Japan’s CEA Scheme: How It Works And Impact So Far
Japan has announced the latest evaluation results under its cost effectiveness assessment scheme. But how does it work and how much have prices been revised so far under the four-year-old program following discussions between pharma firms and authorities?

Moving The Needle In Rare Disease: UCB’s gMG Strategy
Launching a novel drug into a new market is tough. UCB’s Kimberly Moran, talked to In Vivo about how being both patient and digital first gives the company a competitive commercial advantage in an increasingly growing market.

Will Medicare Price Negotiation Stop Post-Approval R&D?
Infographic: One of the more prominent arguments against the Medicare drug price negotiation program is that by limiting the number of years that products can be marketed before price caps are imposed, the scheme discourages development of additional indications.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.