Strategies To Encourage Universal Access To Gene Therapies
Executive Summary
Inaccessible price points, development risk, and a lack of diversity in gene therapy R&D all feed into the imperfect state in which the industry's newest form of innovation exists. New approaches are needed if the patient populations that need these medicines the most can benefit.
It is no secret that the accessibility of gene therapies is a widely debated topic. Therapies continue to be brought to market at inaccessibly high price points, hindering access to the technology for the very patient population the products are designed for.
Safety concerns are creating a relatively early-stage bottleneck in the progress of gene therapies to market. However, the conversation around access needs to start sooner regardless of how nascent the technology is. By having these conversations around lowering the cost per dose early-on, patients can benefit from the therapies sooner and the cost-benefit analysis of these treatments will also improve sooner.
Improved gene therapy cost-benefit may also encourage more development of gene therapies for genetically defined diseases with larger patient populations (non-rare indications, for example) where currently due to the high cost per patient (among other factors such as availability of other treatment options) payers are even less incentivized to reimburse and therefore the market is less attractive for companies.
Negotiating a sustainable pricing model is one element to improving access, but to be sustainable the industry needs to understand how these gene therapies can be produced in a low resource setting, to drive prices down.
When taking this approach, the fact that gene therapy developers are largely focusing their development on high income countries cannot be underestimated. The American Society of Gene and Cell Therapy (ASGCT) conference that took place in May this year was the source of much inspiration on this topic, particularly the talks from Jennifer E. Adair (Fred Hutchinson Cancer Center), Cissy Kityo (Joint Clinical Research Centre), Mike McCune (HIV Frontiers Program), and KV Subramaniam (Reliance Life Sciences) which emphasized the value of putting Low and Middle-Income Countries (LMICs) in the driving seat for the development of this technology.
If gene therapy developers focused on how they could percolate these advanced medical products through LMIC populations and accustom themselves to taking a measure of financial risk, the access hurdles faced in high income countries would be addressed also.
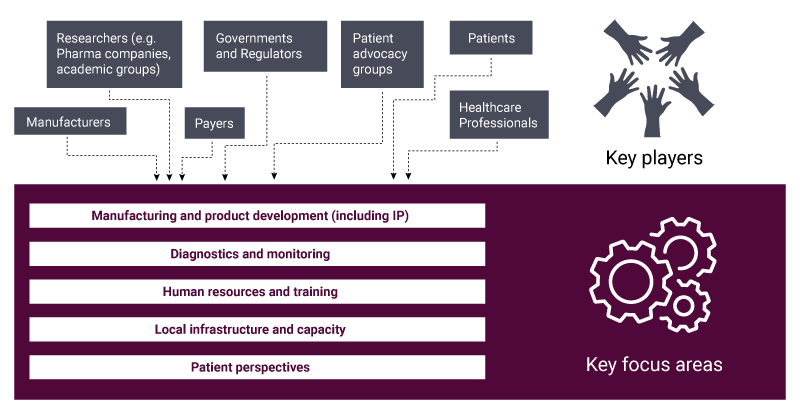
Figure 1 summarizes some of the key factors that need to be considered in the provision of gene therapies, demonstrating the complexities of the various moving parts in the drive of achieving gene therapy access on a global scale, many of which are inter-reliant.
The diagram indicates the hurdles to ensuring the provision of gene therapies is global, and just as viable in LMICs as it could be in High Income Countries (HICs).
While there are nuanced concerns within each category that have been discussed and identified over recent years, there are some underlying themes that, when addressed, could facilitate the path to improvements in access. These themes are a lack of diversity in gene therapy research and development, and the need to incentivize companies to take a risk improve this diversity despite the waters being fairly untouched.
Lack Of Diversity In Gene Therapy R&D
A therapy needs to work in its target population – naturally clinical trials enable investigational products to be tested in patients who have the disease we are aiming to treat, however the current development landscape for gene therapies does not consider geographical/population variations.
Not only is there a stark difference in gene therapy development between HICs such as the US and Western Europe compared to the rest of the world, but more specifically none of the 4,442 gene therapies developed have been trialed in over 90% of African nations.
As pointed out by Jennifer E. Adair at the ASGCT conference, this becomes even more startling when you consider sickle cell disease for which the vast majority of the affected population live in Africa. Zynteglo, approved in the EU in 2019 for Beta Thalassemia but also being developed for sickle cell, has made some of the greatest progress with regards to reimbursement. However, to put this into the context of wider progress in the field, it is being discussed at a threshold price for cost-effectiveness at $2.1m in the US. (Also see "Zynteglo Is Cost Effective At $2.1M, ICER Proposes; Validation For Bluebird’s Pivot To US?" - Scrip, 14 Apr, 2022.)
It would be reductive to suggest this is a disease specific problem, however, considering 84% of the world population live in LMICs, according to the World Bank, and that Africa is the most genetically diverse continent. As such, an LMIC-conscious approach would have benefits not only in terms of the logistical elements of bringing gene therapies to patients at lower costs, but also in ensuring they can be as efficacious and globally applicable as possible. Adair mentioned the Global Gene Therapy Initiative hopes to launch two Phase I gene therapy trials in two LMICs by 2024 – one in India and one in Uganda.
The logistical concerns about how the R&D landscape is structured is founded on the lack of incentives to get the necessary infrastructure and skills in LMICs. When gene therapies get approved and moved to market, deployment in these areas may struggle.
It would be more conducive to improved global access if the resources and skills were in place locally and the technology can grow from within. Designing gene therapies specifically with the challenges of LMICs in the forefront of the manufacturers’ mind (most naturally by developing the gene therapy in the LMICs themselves), will ensure that the technologies are not reliant on resources and ways of thinking only available or applicable to HICs.
The efficacy concerns with the gene therapy R&D landscape relate to how we know therapies are going to work in demographics and ethnicities in which they have not been clinically tested.
Research on the topic has been carried out by the Human Hereditary and Health in Africa (H3 Africa) initiative. Through whole genome sequencing of 426 individuals spanning 50 ethnolinguistic groups throughout Africa, they found “62 previously unreported loci that are under strong selection, which were predominantly found in genes that are involved in viral immunity, DNA repair and metabolism”, all functions which are heavily implicated in the clinical success of gene therapy technology.
“A gene therapy for HIV or sickle cell disease (SCD) that works in Africa will have the greatest transportability around the globe,” said Adair in her ASGCT talk. This is likely to also be the case for other indications as the therapeutic implications of genetic diversity and variation is universal.
Risks And Incentives
Of course, it’s one thing to wish for diversity in gene therapy R&D and another to implement and incentivize players to take the necessary risks. This brings about a chicken-and-egg problem. As Cissy Kityo pointed out in her talk, the way gene therapy R&D is structured currently and the implications that has on price of gene therapies, “it does not make any sense to evaluate such products in clinical trials in populations where access will almost be impossible.”
However, arguably without the locally grown development of gene therapies and associated technologies, access in LMICs will be hard to attain. The cycle needs to be broken to allow the functions in Figure 1 to be present and sustainable in LMICs. As there has been little precedent set in the organic growth of gene therapy development in LMICs, companies and other payers (as described in figure 1) will need to work and think outside of their comfort zone.
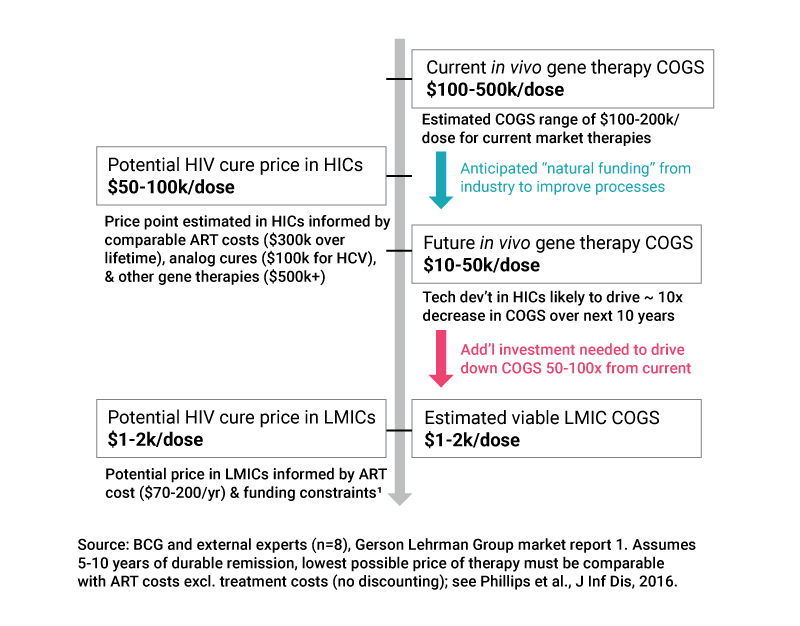
One idea mentioned by HIV Frontiers Program’s Mike McCune in his ASGCT talk "Bringing Safe, Effective, And Accessible Gene Therapies For HIV And Sickle Cell Disease To Resource-Limited Parts Of The World" was a system of tiered amortized payments, with the goal being the cost of gene therapies would only be $1,000-2,000 in sub-Saharan Africa (Figure 2).
In the model proposed, funding and technology development are two of key drivers. In general, funding requires investors to take on an element of risk, and theoretically technology development could be accelerated through freedom of intellectual property (IP), allowing companies who may have new ideas and tools to then partner that with IP from elsewhere and multiplying avenues for next generation technology expansion. With regards to the model McCune suggested, technology development in HICs would likely drive roughly 10x decrease in cost of goods sold (COGS) over a ten year period so this part of the puzzle is not insignificant by any means.
The risk of course lies not only for investors, but freedom surrounding IP would also require a great deal of risk sharing, and outside of gene therapy increasingly we are seeing pharma companies taking more creative and altruistic approaches. Pfizer Inc., for example, have recently announced a non-profit model for sales in 45 lower income countries and ViiV Healthcare have licensed its HIV prevention drug, Apretude, to the Medicines Patent Pool. (Also see "Pfizer To Learn From Pandemic For Health Equity Initiative" - Scrip, 25 May, 2022.)
A concept that is likely to be fundamental for efforts like this to work in gene therapy is having “skin in the game”. McCune mentioned this in the context of pharma companies putting in some of their own money into partnerships, but it’s also something that could work in the context of IP freedom, as a signal to incentivize other players in the market to make a similar move encouraging action and involvement.
On the topic of CRISPR patent pools, for instance, a big concern was whether anyone would input into the pool, or whether instead the vast majority would simply want to reap the benefits of the outputs. However, the steep rise in number of programs entering the pipeline for gene therapies (see Figure 3) should provide hope that candidates would be plentiful. It simply takes a certain number to go first, and a precedent to be set.
Amid the promise of models such as in Figure 3 and progress regarding reimbursement, it is also important to ask whether, even with pricing models designed, most populations in LMICs would benefit due to low healthcare insurance penetration, an idea KV Subramaniam raised in his presentation.
In India, for example, approximately 75% of the population pay for medical services out of their own pocket so any sustainable solution to gene therapy cost will likely need to also take into consideration changes that can be made operationally, or that will remove reliance on payers, which adds further color not only to the importance of driving down gene therapy costs but also focusing efforts at a systemic level in certain countries. Again, the paradigm shift that is required would take time and leaps of faith from therapy developers.
Topics such as improved healthcare insurance penetration and open-source intellectual property are vast areas with incredible complexity, however, they also have positive implications far beyond gene therapy. As such, the incentives for the players in Figure 1 to become involved in tackling these large challenges and building a gene therapy landscape from within LMICs should be even greater, and the financial risks worth taking
It’s an age-old adage, but the message reiterated at the ASGCT 2022 conference was clear: where there is a will, there is a way. The geneticist and former director of the National Human Genome Research Institute, Francis Collins, even presented us with the challenge: "We have to come up with a strategy that would allow treatment, cure even, of SCD in a low resource setting in a one-shot deal that could be affordable. That is a very heavy lift indeed but it's the right kind of lift if we're ambitious about where we want to go with this therapeutic".
Perhaps a most recent example of this is the COVID-19 pandemic, which exemplified that the improvements and efforts we make are a function of the level of urgency we assign to problems, which dictates our incentive to push harder and dig deeper to make a system work. It’s important to peel back the layers and ensure that the foundations we are working with can support the weight of the huge potential of these life-changing therapies.